Retinoic acid receptor
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
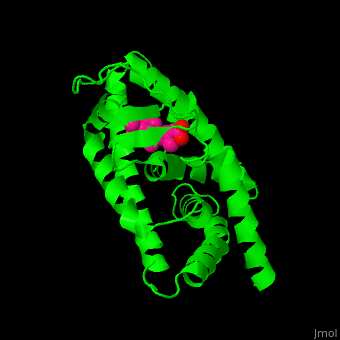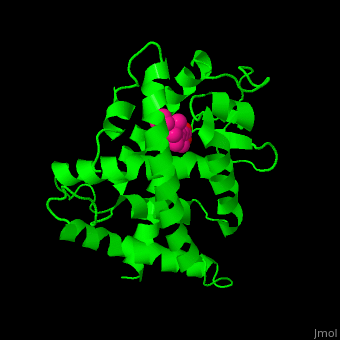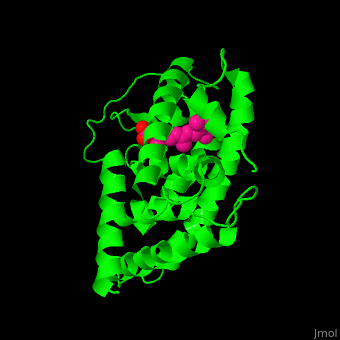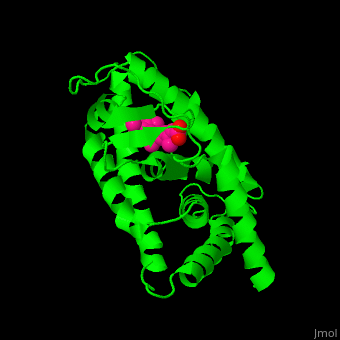
<StructureSection load='' size='350' side='right' caption='Structure of human retinoic acid receptor γ ligand-binding domain complex with all-trans retinoic acid (PDB entry [[2lbd]])' scene='51/516466/Cv/3'> | <StructureSection load='' size='350' side='right' caption='Structure of human retinoic acid receptor γ ligand-binding domain complex with all-trans retinoic acid (PDB entry [[2lbd]])' scene='51/516466/Cv/3'> | ||
== Function == | == Function == | ||
| - | '''Retinoic acid receptor''' (RAR) is a [[Nuclear receptors|nuclear receptor]] activated by all-''trans'' and 9-''cis'' retinoic acid. RAR makes a heterodimer with retinoid X receptor (RXR). In the absence of ligand, the RAR/RXR complex binds the hormone response elements complexed with corepressor protein. Upon binding of the ligand, the corepressor disassociates from the receptor and associates with the coactivator leading to transcription activation. RAR contains a DNA-binding domain (DBD) and ligand-binding domain (LBD). There are 3 classes of RAR: α, β and γ. | + | '''Retinoic acid receptor''' (RAR) is a [[Nuclear receptors|nuclear receptor]] activated by all-''trans'' and 9-''cis'' retinoic acid. RAR makes a heterodimer with '''retinoid X receptor''' (RXR). In the absence of ligand, the RAR/RXR complex binds the hormone response elements complexed with corepressor protein. Upon binding of the ligand, the corepressor disassociates from the receptor and associates with the coactivator leading to transcription activation. RAR contains a DNA-binding domain (DBD) and ligand-binding domain (LBD). There are 3 classes of RAR: α, β and γ. |
*'''RARα''' plays an important role in mediating all-transretinoic acid signals<ref>PMID:15171703</ref>.<BR /> | *'''RARα''' plays an important role in mediating all-transretinoic acid signals<ref>PMID:15171703</ref>.<BR /> | ||
Revision as of 07:11, 12 October 2023
| |||||||||||
References
- ↑ Wu Q, Lin XF, Ye XF, Zhang B, Xie Z, Su WJ. Ubiquitinated or sumoylated retinoic acid receptor alpha determines its characteristic and interacting model with retinoid X receptor alpha in gastric and breast cancer cells. J Mol Endocrinol. 2004 Jun;32(3):595-613. PMID:15171703
- ↑ Sommer KM, Chen LI, Treuting PM, Smith LT, Swisshelm K. Elevated retinoic acid receptor beta(4) protein in human breast tumor cells with nuclear and cytoplasmic localization. Proc Natl Acad Sci U S A. 1999 Jul 20;96(15):8651-6. PMID:10411930
- ↑ Johansson HJ, Sanchez BC, Mundt F, Forshed J, Kovacs A, Panizza E, Hultin-Rosenberg L, Lundgren B, Martens U, Mathe G, Yakhini Z, Helou K, Krawiec K, Kanter L, Hjerpe A, Stal O, Linderholm BK, Lehtio J. Retinoic acid receptor alpha is associated with tamoxifen resistance in breast cancer. Nat Commun. 2013;4:2175. doi: 10.1038/ncomms3175. PMID:23868472 doi:http://dx.doi.org/10.1038/ncomms3175
- ↑ Khuri FR, Lotan R, Kemp BL, Lippman SM, Wu H, Feng L, Lee JJ, Cooksley CS, Parr B, Chang E, Walsh GL, Lee JS, Hong WK, Xu XC. Retinoic acid receptor-beta as a prognostic indicator in stage I non-small-cell lung cancer. J Clin Oncol. 2000 Aug;18(15):2798-804. PMID:10920126
- ↑ Soprano KJ, Soprano DR. Retinoic acid receptors and cancer. J Nutr. 2002 Dec;132(12):3809S-3813S. PMID:12468629
- ↑ Renaud JP, Rochel N, Ruff M, Vivat V, Chambon P, Gronemeyer H, Moras D. Crystal structure of the RAR-gamma ligand-binding domain bound to all-trans retinoic acid. Nature. 1995 Dec 14;378(6558):681-9. PMID:7501014 doi:http://dx.doi.org/10.1038/378681a0