We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Ribavirin1
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
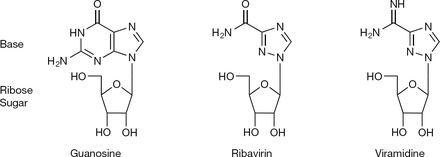
1-β-D-ribofuranosyl-1H-1,2,4-triazole-3-carboxamide <ref name="gish">DOI: 10.1093/jac/dki405 </ref> | 1-β-D-ribofuranosyl-1H-1,2,4-triazole-3-carboxamide <ref name="gish">DOI: 10.1093/jac/dki405 </ref> | ||
<StructureSection load='' size='340' side='right' caption='Ribavirin PDB [[4pb1]]' scene='74/746008/Ribavirin_atalla2/1'> | <StructureSection load='' size='340' side='right' caption='Ribavirin PDB [[4pb1]]' scene='74/746008/Ribavirin_atalla2/1'> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
== Overview == | == Overview == | ||
Ribavirin was first synthesized in 1970 by ICN Pharmaceuticals (now “Valent International Pharmaceuticals”). In 1986, its first major use was the treatment of RSV (respiratory syncitial virus) infections in pediatric patients, but since its FDA approval in 1998, it has primarily been used as a component in treating Hepatitis C by inhibiting the synthesis of viral RNA. <ref>https://pubchem.ncbi.nlm.nih.gov/compound/37542<ref> The treatment was modified and approved in 2002 by the FDA by combining it with interferon alfa2b. <ref name="gish"/> | Ribavirin was first synthesized in 1970 by ICN Pharmaceuticals (now “Valent International Pharmaceuticals”). In 1986, its first major use was the treatment of RSV (respiratory syncitial virus) infections in pediatric patients, but since its FDA approval in 1998, it has primarily been used as a component in treating Hepatitis C by inhibiting the synthesis of viral RNA. <ref>https://pubchem.ncbi.nlm.nih.gov/compound/37542<ref> The treatment was modified and approved in 2002 by the FDA by combining it with interferon alfa2b. <ref name="gish"/> | ||
Revision as of 14:52, 4 January 2024
1-β-D-ribofuranosyl-1H-1,2,4-triazole-3-carboxamide [1]
| |||||||||||
References
- Gish, R. G. Treating HCV with ribavirin analogue and ribavirin-like molecules. Journal of Antimicrobial Chemotherapy. 2005, November 17;1-6. doi:10.1093/jac/dki405
- Thomas, E., Ghany, M., Liang, J., The application and mechanism of action of ribavirin in therapy of hepatitis C. Antiviral Chemistry & Chemotherapy 23:1-12 (2012)doi: 10.3851/IMP2125
- Chung, R.T., Gale, M.J., Polyak, S.J., Lemon, S.M., Liang, T.J., & Hoofnagle, J.H. Mechanisms of action of interferon and ribavirin in chronic hepatitis C: Summary of a workshop. Hepatology. 2008;47 (1), 306-320. doi: 10.1002/hep.22070
- Paeshuyse, J, Dallmeier, K, Neyts, J. Ribavirin for the treatment of chronic hepatitis C virus infection: a review of the proposed mechanisms of action. Current Opinion in Virology. 2011;1(6) 590-598. doi: 10.1016/j.coviro.2011.10.030
- National Heart, Lung and Blood Institute. Pneumonia. 2016, September 26; Retrieved from https://www.nhlbi.nih.gov/health/health-topics/topics/pnu
- Foster, G. Pegylated interferons for the treatment of chronic Hepatitis C. Drugs. 2010;70(2):147-165. doi:10.2165/11531990-000000000-00000
- Hofmann WP, Herrmann E, Sarrazin C, Zeuzem S. Ribavirin mode of action in chronic hepatitis C: from clinical use back to molecular mechanisms. Liver Int. 2008, 28:1332-1343. doi: 10.1111/j.1478-3231.2008.01896.x
- Alam, I., Lee, J., Cho, K. J., Han, K.R., Yang, J.M., Chung, M.S., & Kim, K. H. Crystal structures of murine norovirus-1 RNA-dependent RNA polymerase in complex with 2-thiouridine or ribavirin. Virology. 2012, 426: 143-151. http://dx.doi.org/10.1016/j.virol.2012.01.016
- Johnson, ZL, Lee, JH, Lee, M, Kwon, DY, Hong, J, Lee, SY. Structural basis of nucleoside and nucleoside drug selectivity by concentrative nucleoside transporters. eLife. 2014, 3:e03604. doi: 10.7554/eLife.03604.
- Fujimoto T, Tomimatsu M, Iga D, Endo H, Otsuka K. Changes in the Th1/Th2 ratio during a 24-week course of an interferon alpha-2b plus ribavirin combination therapy for patients with chronic hepatitis C. J. Gastroenterol. Hepatol. 2008, 23:E432- E437. doi: 10.1111/j.1440-1746.2008.05320.x
- Feld, JJ, Nanda, S, Huang, Y, Chen, W, Cam, M, Pusek, SN, Schwigler, LM, Theodore, D, Zacks, SL, Liang, TJ, Fried, MW. Hepatic gene expression during treatment with peginterferon and ribavirin: Identifying molecule pathways for treatment response. Hepatol. 2007, 46(5): 1548-1563. doi: 10.1002/hep.21853
- Thomas E, Feld JJ, Li QS, Hu ZY, Fried MW, Liang TJ. Ribavirin potentiates interferon action by augmenting interferon stimulated gene induction in hepatitis C virus cell culture models. Hepatology 2011, 53:32-41. doi: 10.1002/hep.23985
- Shu QN, Nair V. Inosine monophosphate dehydrogenase (IMPDH) as a target in drug discovery. Med. Res. Rev. 2008, 28:219-232. doi: 10.1002/chin.200823265
- Streeter, DG, Witkowski, JT, Khare, GP, Sidwell, RW, Bauer, RJ, Robins, RK, Simon, LN. Mechanisms of action of 1-β-D-ribofuranosyl-1,2,4-triazole-3-carboxamide (virazole) a new broad spectrum antiviral agent. Proc. Natl. Acad. Sci. 1973, 70:1174-1178. PMID: 4197928
- Kentsis, A, Topisirovic, I, Culjkovic, B, Shao, L, Borden, KLB. Ribavirin suppresses eIF4E-mediated oncogenic transformation by physical mimicry of the guanosine mRNA cap. Proc. Natl. Acad. Sci. 2004, 101:18105-18110. doi: 10.1073/pnas.0406927102
- Graci, JD, Cameron, CE. Mechanisms of action of ribavirin against distinct viruses. Rev. Med. Virol. 2006, 16: 37-48. doi: 10.1002/rmv.483