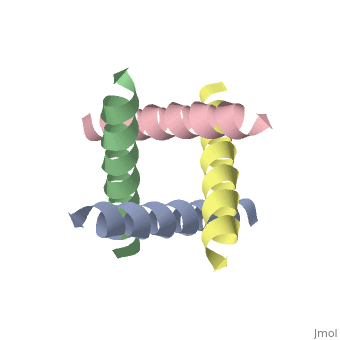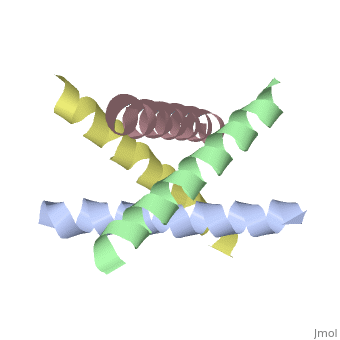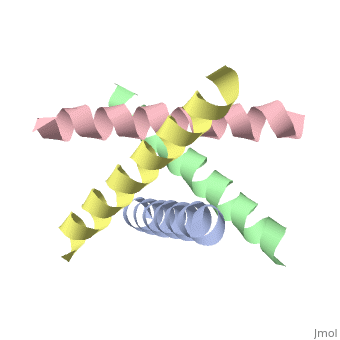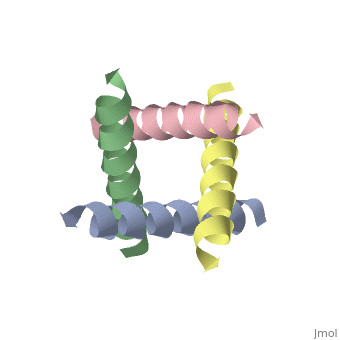We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
M2 Proton Channel
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| + | <StructureSection load='1nyj' size='340' side='right' caption='The closed state structure of M2 protein H+ channel by solid state NMR spectroscopy ([[1nyj]])' scene=''> | ||
== M2 Proton Channel from ''Influenza'' A Virus == | == M2 Proton Channel from ''Influenza'' A Virus == | ||
| - | <applet load='1nyj' size='300' frame='true' align='right' caption='The closed state structure of M2 protein H+ channel by solid state NMR spectroscopy. [Stouffer et al, 2008] [[1nyj]]' /> | ||
== Background == | == Background == | ||
The M2 proton channel is a key protein that leads to viral infection.<ref name="Takeuchi" /> The M2 proton channel acidifies the virion which allows the viral matrix protein (M1) to disassociate from the ribonucleoprotein (RNP).<ref name="Wu">PMID:12972147 </ref> This allows the RNP to be transported to the nucleus of the cell. Several recent studies have looked at the effects of <scene name='User:Sarah_Henke/Sandbox_1/Amantadine/1'>amantadine</scene> ([[Symmetrel]])<ref name="Stouffer">PMID:18235504 </ref> and <scene name='User:Sarah_Henke/Sandbox_1/Rimantadine/1'>rimantadine</scene> ([[Flumadine]])<ref name="Schnell">PMID:18235503 </ref> on inhibiting the transfer of protons through the M2 channel.<ref name="Stouffer" /> Amantadine is a proton surrogate that competes with protons for binding to His37, the residue involved in the gating mechanism.<ref name="Lear" /><ref>PMID:3662473</ref><ref>PMID:17156962</ref> It has been found that M2 is resistant to these two drugs in 90% of humans, birds and pigs. Understanding the structure and function of this proton channel is necessary in solving the resistance problem.<ref name="Stouffer" /> | The M2 proton channel is a key protein that leads to viral infection.<ref name="Takeuchi" /> The M2 proton channel acidifies the virion which allows the viral matrix protein (M1) to disassociate from the ribonucleoprotein (RNP).<ref name="Wu">PMID:12972147 </ref> This allows the RNP to be transported to the nucleus of the cell. Several recent studies have looked at the effects of <scene name='User:Sarah_Henke/Sandbox_1/Amantadine/1'>amantadine</scene> ([[Symmetrel]])<ref name="Stouffer">PMID:18235504 </ref> and <scene name='User:Sarah_Henke/Sandbox_1/Rimantadine/1'>rimantadine</scene> ([[Flumadine]])<ref name="Schnell">PMID:18235503 </ref> on inhibiting the transfer of protons through the M2 channel.<ref name="Stouffer" /> Amantadine is a proton surrogate that competes with protons for binding to His37, the residue involved in the gating mechanism.<ref name="Lear" /><ref>PMID:3662473</ref><ref>PMID:17156962</ref> It has been found that M2 is resistant to these two drugs in 90% of humans, birds and pigs. Understanding the structure and function of this proton channel is necessary in solving the resistance problem.<ref name="Stouffer" /> | ||
| Line 32: | Line 32: | ||
*[[Treatments:M2 Proton Channel Inhibitor Pharmacokinetics]]<br /> | *[[Treatments:M2 Proton Channel Inhibitor Pharmacokinetics]]<br /> | ||
*[[Treatments:Influenza]]. | *[[Treatments:Influenza]]. | ||
| - | + | </StructureSection> | |
== References == | == References == | ||
<references /> | <references /> | ||
Current revision
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Takeuchi H, Okada A, Miura T. Roles of the histidine and tryptophan side chains in the M2 proton channel from influenza A virus. FEBS Lett. 2003 Sep 18;552(1):35-8. PMID:12972149
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 Wu Y, Voth GA. Computational studies of proton transport through the M2 channel. FEBS Lett. 2003 Sep 18;552(1):23-7. PMID:12972147
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Stouffer AL, Acharya R, Salom D, Levine AS, Di Costanzo L, Soto CS, Tereshko V, Nanda V, Stayrook S, DeGrado WF. Structural basis for the function and inhibition of an influenza virus proton channel. Nature. 2008 Jan 31;451(7178):596-9. PMID:18235504 doi:10.1038/nature06528
- ↑ Schnell JR, Chou JJ. Structure and mechanism of the M2 proton channel of influenza A virus. Nature. 2008 Jan 31;451(7178):591-5. PMID:18235503 doi:10.1038/nature06531
- ↑ 5.0 5.1 5.2 5.3 Lear JD. Proton conduction through the M2 protein of the influenza A virus; a quantitative, mechanistic analysis of experimental data. FEBS Lett. 2003 Sep 18;552(1):17-22. PMID:12972146
- ↑ Anderson EL, Van Voris LP, Bartram J, Hoffman HE, Belshe RB. Pharmacokinetics of a single dose of rimantadine in young adults and children. Antimicrob Agents Chemother. 1987 Jul;31(7):1140-2. PMID:3662473
- ↑ Wang P, Liang YZ, Chen BM, Zhou N, Yi LZ, Yu Y, Yi ZB. Quantitative determination of amantadine in human plasma by liquid chromatography-mass spectrometry and the application in a bioequivalence study. J Pharm Biomed Anal. 2007 Mar 12;43(4):1519-25. Epub 2006 Dec 6. PMID:17156962 doi:10.1016/j.jpba.2006.10.044
Proteopedia Page Contributors and Editors (what is this?)
Sarah Henke, David Canner, Michal Harel, Alexander Berchansky, Eric Martz