1rh2
From Proteopedia
OCA (Talk | contribs)
(New page: 200px<br /> <applet load="1rh2" size="450" color="white" frame="true" align="right" spinBox="true" caption="1rh2, resolution 2.9Å" /> '''RECOMBINANT HUMAN IN...)
Next diff →
Revision as of 16:57, 12 November 2007
|
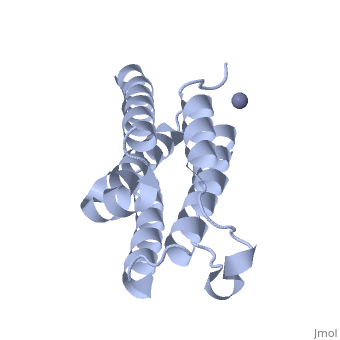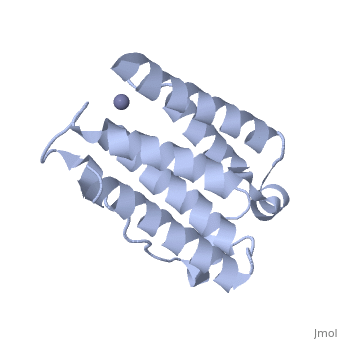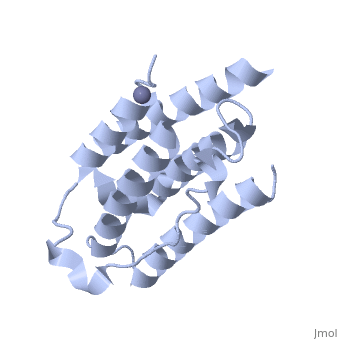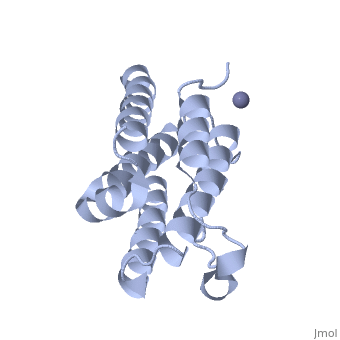
RECOMBINANT HUMAN INTERFERON-ALPHA 2B
Overview
BACKGROUND: The human alpha-interferon (huIFN-alpha) family displays broad, spectrum antiviral, antiproliferative and immunomodulatory activities on a, variety of cell types. The diverse biological activities of the, IFN-alpha's are conveyed to cells through specific interactions with, cell-surface receptors. Despite considerable effort, no crystal structure, of a member of this family has yet been reported, because the quality of, the protein crystals have been unsuitable for crystallographic studies., Until now, structural models of the IFN-alpha's have been based on the, structure of murine IFN-beta (muIFN-beta). These models are likely to be, inaccurate, as the amino acid sequence of muIFN-beta differs significantly, from the IFN-alpha's at proposed receptor-binding sites. Structural, information on a huIFN-alpha subtype would provide an improved basis for, modeling the structures of the entire IFN-alpha family. RESULTS: The, crystal structure of recombinant human interferon-alpha 2b (huIFN-alpha, 2b) has been determined at 2.9 A resolution. HuIFN-alpha 2b exists in the, crystal as a noncovalent dimer, which associates in a novel manner. Unlike, other structurally characterized cytokines, extensive interactions in the, dimer interface are mediated by a zinc ion (Zn2+). The overall fold of, huIFN-alpha 2b is most similar to the structure of muIFN-beta. Unique to, huIFN-alpha 2b is a 3(10) helix in the AB loop which is held to the core, of the molecule by a disulfide bond. CONCLUSIONS: The structure of, huIFN-alpha 2b provides an accurate model for analysis of the > 15 related, type 1 interferon molecules. HuIFN-alpha 2b displays considerable, structural similarity with muIFN-beta, interleukin-10 and, interferon-gamma, which also bind related class 2 cytokine receptors. From, these structural comparisons and numerous studies on the effects of, mutations on biological activity, we have identified protein surfaces that, appear to be important in receptor activation. This study also reveals the, potential biological importance of the huIFN-alpha 2b dimer.
About this Structure
1RH2 is a Single protein structure of sequence from Homo sapiens with ZN as ligand. Full crystallographic information is available from OCA.
Reference
Zinc mediated dimer of human interferon-alpha 2b revealed by X-ray crystallography., Radhakrishnan R, Walter LJ, Hruza A, Reichert P, Trotta PP, Nagabhushan TL, Walter MR, Structure. 1996 Dec 15;4(12):1453-63. PMID:8994971
Page seeded by OCA on Mon Nov 12 19:03:38 2007