We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
1cma
From Proteopedia
(Difference between revisions)
| Line 4: | Line 4: | ||
== Structural highlights == | == Structural highlights == | ||
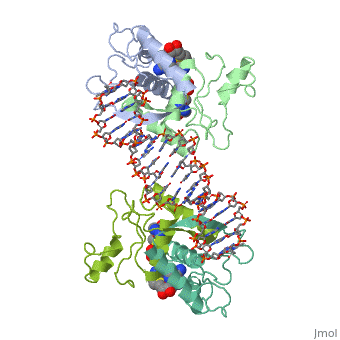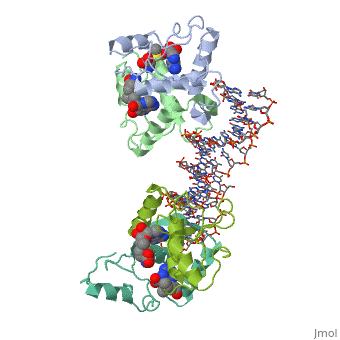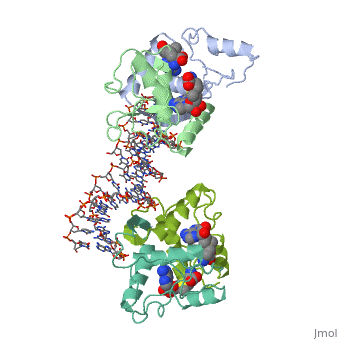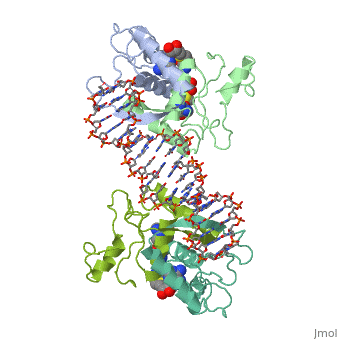
<table><tr><td colspan='2'>[[1cma]] is a 4 chain structure with sequence from [https://en.wikipedia.org/wiki/Escherichia_coli Escherichia coli]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1CMA OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=1CMA FirstGlance]. <br> | <table><tr><td colspan='2'>[[1cma]] is a 4 chain structure with sequence from [https://en.wikipedia.org/wiki/Escherichia_coli Escherichia coli]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1CMA OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=1CMA FirstGlance]. <br> | ||
| - | </td></tr><tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=SAM:S-ADENOSYLMETHIONINE'>SAM</scene></td></tr> | + | </td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 2.8Å</td></tr> |
| + | <tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=SAM:S-ADENOSYLMETHIONINE'>SAM</scene></td></tr> | ||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=1cma FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1cma OCA], [https://pdbe.org/1cma PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=1cma RCSB], [https://www.ebi.ac.uk/pdbsum/1cma PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=1cma ProSAT]</span></td></tr> | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=1cma FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1cma OCA], [https://pdbe.org/1cma PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=1cma RCSB], [https://www.ebi.ac.uk/pdbsum/1cma PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=1cma ProSAT]</span></td></tr> | ||
</table> | </table> | ||
== Function == | == Function == | ||
| - | + | [https://www.uniprot.org/uniprot/METJ_ECOLI METJ_ECOLI] This regulatory protein, when combined with SAM (S-adenosylmethionine) represses the expression of the methionine regulon and of enzymes involved in SAM synthesis. It is also autoregulated.[HAMAP-Rule:MF_00744] | |
== Evolutionary Conservation == | == Evolutionary Conservation == | ||
[[Image:Consurf_key_small.gif|200px|right]] | [[Image:Consurf_key_small.gif|200px|right]] | ||
| Line 19: | Line 20: | ||
</jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=1cma ConSurf]. | </jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=1cma ConSurf]. | ||
<div style="clear:both"></div> | <div style="clear:both"></div> | ||
| - | <div style="background-color:#fffaf0;"> | ||
| - | == Publication Abstract from PubMed == | ||
| - | The crystal structure of the met repressor-operator complex shows two dimeric repressor molecules bound to adjacent sites 8 base pairs apart on an 18-base-pair DNA fragment. Sequence specificity is achieved by insertion of double-stranded antiparallel protein beta-ribbons into the major groove of B-form DNA, with direct hydrogen-bonding between amino-acid side chains and the base pairs. The repressor also recognizes sequence-dependent distortion or flexibility of the operator phosphate backbone, conferring specificity even for inaccessible base pairs. | ||
| - | |||
| - | Crystal structure of the met repressor-operator complex at 2.8 A resolution reveals DNA recognition by beta-strands.,Somers WS, Phillips SE Nature. 1992 Oct 1;359(6394):387-93. PMID:1406951<ref>PMID:1406951</ref> | ||
| - | |||
| - | From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | ||
| - | </div> | ||
| - | <div class="pdbe-citations 1cma" style="background-color:#fffaf0;"></div> | ||
==See Also== | ==See Also== | ||
*[[Met repressor|Met repressor]] | *[[Met repressor|Met repressor]] | ||
| - | == References == | ||
| - | <references/> | ||
__TOC__ | __TOC__ | ||
</StructureSection> | </StructureSection> | ||
[[Category: Escherichia coli]] | [[Category: Escherichia coli]] | ||
[[Category: Large Structures]] | [[Category: Large Structures]] | ||
| - | [[Category: Phillips | + | [[Category: Phillips SEV]] |
| - | [[Category: Somers | + | [[Category: Somers WS]] |
| - | + | ||
| - | + | ||
| - | + | ||
Current revision
MET REPRESSOR/DNA COMPLEX + S-ADENOSYL-METHIONINE
| |||||||||||