This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
1js3
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
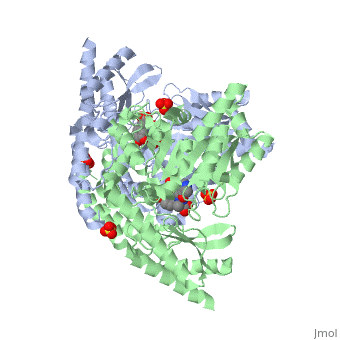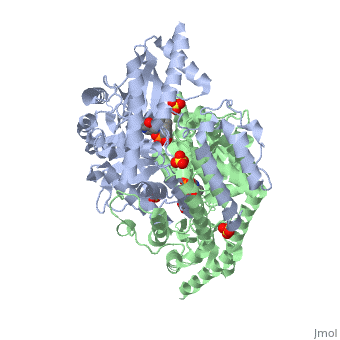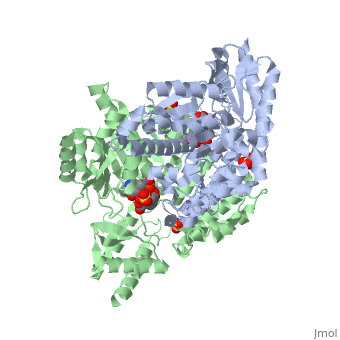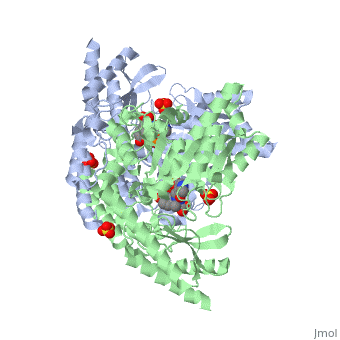
<StructureSection load='1js3' size='340' side='right'caption='[[1js3]], [[Resolution|resolution]] 2.25Å' scene=''> | <StructureSection load='1js3' size='340' side='right'caption='[[1js3]], [[Resolution|resolution]] 2.25Å' scene=''> | ||
== Structural highlights == | == Structural highlights == | ||
| - | <table><tr><td colspan='2'>[[1js3]] is a 2 chain structure with sequence from [https://en.wikipedia.org/wiki/ | + | <table><tr><td colspan='2'>[[1js3]] is a 2 chain structure with sequence from [https://en.wikipedia.org/wiki/Sus_scrofa Sus scrofa]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1JS3 OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=1JS3 FirstGlance]. <br> |
| - | </td></tr><tr id=' | + | </td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 2.25Å</td></tr> |
| - | <tr id=' | + | <tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=142:CARBIDOPA'>142</scene>, <scene name='pdbligand=PLP:PYRIDOXAL-5-PHOSPHATE'>PLP</scene>, <scene name='pdbligand=SO4:SULFATE+ION'>SO4</scene></td></tr> |
| - | + | ||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=1js3 FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1js3 OCA], [https://pdbe.org/1js3 PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=1js3 RCSB], [https://www.ebi.ac.uk/pdbsum/1js3 PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=1js3 ProSAT]</span></td></tr> | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=1js3 FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1js3 OCA], [https://pdbe.org/1js3 PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=1js3 RCSB], [https://www.ebi.ac.uk/pdbsum/1js3 PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=1js3 ProSAT]</span></td></tr> | ||
</table> | </table> | ||
== Function == | == Function == | ||
| - | + | [https://www.uniprot.org/uniprot/DDC_PIG DDC_PIG] Catalyzes the decarboxylation of L-3,4-dihydroxyphenylalanine (DOPA) to dopamine, L-5-hydroxytryptophan to serotonin and L-tryptophan to tryptamine. | |
== Evolutionary Conservation == | == Evolutionary Conservation == | ||
[[Image:Consurf_key_small.gif|200px|right]] | [[Image:Consurf_key_small.gif|200px|right]] | ||
| Line 21: | Line 20: | ||
</jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=1js3 ConSurf]. | </jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=1js3 ConSurf]. | ||
<div style="clear:both"></div> | <div style="clear:both"></div> | ||
| - | <div style="background-color:#fffaf0;"> | ||
| - | == Publication Abstract from PubMed == | ||
| - | DOPA decarboxylase (DDC) is responsible for the synthesis of the key neurotransmitters dopamine and serotonin via decarboxylation of L-3,4-dihydroxyphenylalanine (L-DOPA) and L-5-hydroxytryptophan, respectively. DDC has been implicated in a number of clinic disorders, including Parkinson's disease and hypertension. Peripheral inhibitors of DDC are currently used to treat these diseases. We present the crystal structures of ligand-free DDC and its complex with the anti-Parkinson drug carbiDOPA. The inhibitor is bound to the enzyme by forming a hydrazone linkage with the cofactor, and its catechol ring is deeply buried in the active site cleft. The structures provide the molecular basis for the development of new inhibitors of DDC with better pharmacological characteristics. | ||
| - | |||
| - | Structural insight into Parkinson's disease treatment from drug-inhibited DOPA decarboxylase.,Burkhard P, Dominici P, Borri-Voltattorni C, Jansonius JN, Malashkevich VN Nat Struct Biol. 2001 Nov;8(11):963-7. PMID:11685243<ref>PMID:11685243</ref> | ||
| - | |||
| - | From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | ||
| - | </div> | ||
| - | <div class="pdbe-citations 1js3" style="background-color:#fffaf0;"></div> | ||
==See Also== | ==See Also== | ||
*[[DOPA decarboxylase|DOPA decarboxylase]] | *[[DOPA decarboxylase|DOPA decarboxylase]] | ||
| - | == References == | ||
| - | <references/> | ||
__TOC__ | __TOC__ | ||
</StructureSection> | </StructureSection> | ||
| - | [[Category: Aromatic-L-amino-acid decarboxylase]] | ||
[[Category: Large Structures]] | [[Category: Large Structures]] | ||
| - | [[Category: | + | [[Category: Sus scrofa]] |
| - | [[Category: Borri-Voltattorni | + | [[Category: Borri-Voltattorni C]] |
| - | [[Category: Burkhard | + | [[Category: Burkhard P]] |
| - | [[Category: Dominici | + | [[Category: Dominici P]] |
| - | [[Category: Jansonius | + | [[Category: Jansonius JN]] |
| - | [[Category: Malashkevich | + | [[Category: Malashkevich VN]] |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
Current revision
Crystal structure of dopa decarboxylase in complex with the inhibitor carbidopa
| |||||||||||