We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
3lc6
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
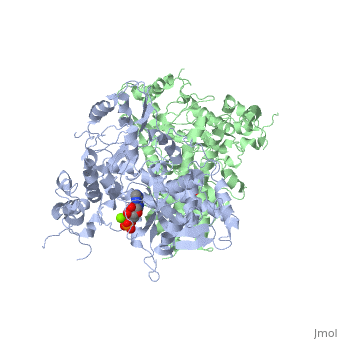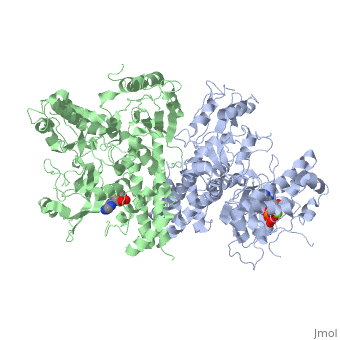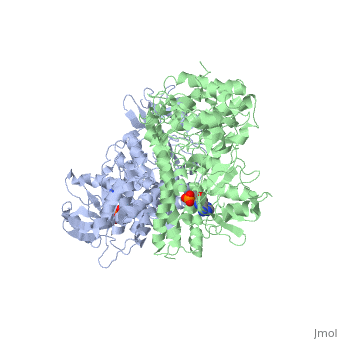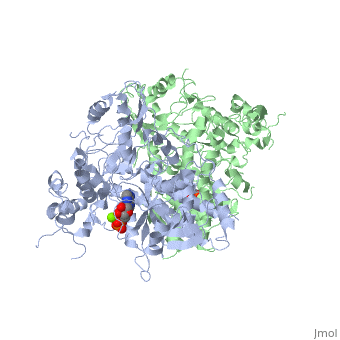
==The alternative conformation structure of isocitrate dehydrogenase kinase/phosphatase from E. Coli== | ==The alternative conformation structure of isocitrate dehydrogenase kinase/phosphatase from E. Coli== | ||
| - | <StructureSection load='3lc6' size='340' side='right' caption='[[3lc6]], [[Resolution|resolution]] 3.10Å' scene=''> | + | <StructureSection load='3lc6' size='340' side='right'caption='[[3lc6]], [[Resolution|resolution]] 3.10Å' scene=''> |
== Structural highlights == | == Structural highlights == | ||
| - | <table><tr><td colspan='2'>[[3lc6]] is a 2 chain structure with sequence from [ | + | <table><tr><td colspan='2'>[[3lc6]] is a 2 chain structure with sequence from [https://en.wikipedia.org/wiki/Escherichia_coli_O157:H7 Escherichia coli O157:H7]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=3LC6 OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=3LC6 FirstGlance]. <br> |
| - | </td></tr><tr id=' | + | </td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 3.1Å</td></tr> |
| - | <tr id=' | + | <tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=ADP:ADENOSINE-5-DIPHOSPHATE'>ADP</scene>, <scene name='pdbligand=AMP:ADENOSINE+MONOPHOSPHATE'>AMP</scene>, <scene name='pdbligand=MG:MAGNESIUM+ION'>MG</scene></td></tr> |
| - | < | + | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=3lc6 FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=3lc6 OCA], [https://pdbe.org/3lc6 PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=3lc6 RCSB], [https://www.ebi.ac.uk/pdbsum/3lc6 PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=3lc6 ProSAT]</span></td></tr> |
| - | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[ | + | |
</table> | </table> | ||
== Function == | == Function == | ||
| - | [ | + | [https://www.uniprot.org/uniprot/ACEK_ECO57 ACEK_ECO57] Bifunctional enzyme which can phosphorylate or dephosphorylate isocitrate dehydrogenase (IDH) on a specific serine residue. This is a regulatory mechanism which enables bacteria to bypass the Krebs cycle via the glyoxylate shunt in response to the source of carbon. When bacteria are grown on glucose, IDH is fully active and unphosphorylated, but when grown on acetate or ethanol, the activity of IDH declines drastically concomitant with its phosphorylation. |
== Evolutionary Conservation == | == Evolutionary Conservation == | ||
[[Image:Consurf_key_small.gif|200px|right]] | [[Image:Consurf_key_small.gif|200px|right]] | ||
| Line 21: | Line 20: | ||
</jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=3lc6 ConSurf]. | </jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=3lc6 ConSurf]. | ||
<div style="clear:both"></div> | <div style="clear:both"></div> | ||
| - | <div style="background-color:#fffaf0;"> | ||
| - | == Publication Abstract from PubMed == | ||
| - | The Escherichia coli isocitrate dehydrogenase kinase/phosphatase (AceK) is a unique bifunctional enzyme that phosphorylates or dephosphorylates isocitrate dehydrogenase (ICDH) in response to environmental changes, resulting in the inactivation or, respectively, activation of ICDH. ICDH inactivation short-circuits the Krebs cycle by enabling the glyoxlate bypass. It was the discovery of AceK and ICDH that established the existence of protein phosphorylation regulation in prokaryotes. As a 65-kDa protein, AceK is significantly larger than typical eukaryotic protein kinases. Apart from the ATP-binding motif, AceK does not share sequence homology with any eukaryotic protein kinase or phosphatase. Most intriguingly, AceK possesses the two opposing activities of protein kinase and phosphatase within one protein, and specifically recognizes only intact ICDH. Additionally, AceK has strong ATPase activity. It has been shown that AceK kinase, phosphatase and ATPase activities reside at the same site, although the molecular basis of such multifunctionality and its regulation remains completely unknown. Here we report the structures of AceK and its complex with ICDH. The AceK structure reveals a eukaryotic protein-kinase-like domain containing ATP and a regulatory domain with a novel fold. As an AceK phosphatase activator and kinase inhibitor, AMP is found to bind in an allosteric site between the two AceK domains. An AMP-mediated conformational change exposes and shields ATP, acting as a switch between AceK kinase and phosphatase activities, and ICDH-binding induces further conformational change for AceK activation. The substrate recognition loop of AceK binds to the ICDH dimer, allowing higher-order substrate recognition and interaction, and inducing critical conformational change at the phosphorylation site of ICDH. | ||
| - | |||
| - | Structure of the bifunctional isocitrate dehydrogenase kinase/phosphatase.,Zheng J, Jia Z Nature. 2010 Jun 17;465(7300):961-5. Epub 2010 May 26. PMID:20505668<ref>PMID:20505668</ref> | ||
| - | |||
| - | From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | ||
| - | </div> | ||
| - | <div class="pdbe-citations 3lc6" style="background-color:#fffaf0;"></div> | ||
==See Also== | ==See Also== | ||
*[[Isocitrate dehydrogenase kinase/phosphatase|Isocitrate dehydrogenase kinase/phosphatase]] | *[[Isocitrate dehydrogenase kinase/phosphatase|Isocitrate dehydrogenase kinase/phosphatase]] | ||
| - | == References == | ||
| - | <references/> | ||
__TOC__ | __TOC__ | ||
</StructureSection> | </StructureSection> | ||
| - | [[Category: | + | [[Category: Escherichia coli O157:H7]] |
| - | + | [[Category: Large Structures]] | |
| - | [[Category: | + | [[Category: Jia Z]] |
| - | [[Category: | + | [[Category: Zheng J]] |
| - | [[Category: | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
Current revision
The alternative conformation structure of isocitrate dehydrogenase kinase/phosphatase from E. Coli
| |||||||||||