We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
5jk7
From Proteopedia
(Difference between revisions)
| Line 9: | Line 9: | ||
== Function == | == Function == | ||
[https://www.uniprot.org/uniprot/DDB1_HUMAN DDB1_HUMAN] Required for DNA repair. Binds to DDB2 to form the UV-damaged DNA-binding protein complex (the UV-DDB complex). The UV-DDB complex may recognize UV-induced DNA damage and recruit proteins of the nucleotide excision repair pathway (the NER pathway) to initiate DNA repair. The UV-DDB complex preferentially binds to cyclobutane pyrimidine dimers (CPD), 6-4 photoproducts (6-4 PP), apurinic sites and short mismatches. Also appears to function as a component of numerous distinct DCX (DDB1-CUL4-X-box) E3 ubiquitin-protein ligase complexes which mediate the ubiquitination and subsequent proteasomal degradation of target proteins. The functional specificity of the DCX E3 ubiquitin-protein ligase complex is determined by the variable substrate recognition component recruited by DDB1. DCX(DDB2) (also known as DDB1-CUL4-ROC1, CUL4-DDB-ROC1 and CUL4-DDB-RBX1) may ubiquitinate histone H2A, histone H3 and histone H4 at sites of UV-induced DNA damage. The ubiquitination of histones may facilitate their removal from the nucleosome and promote subsequent DNA repair. DCX(DDB2) also ubiquitinates XPC, which may enhance DNA-binding by XPC and promote NER. DCX(DTL) plays a role in PCNA-dependent polyubiquitination of CDT1 and MDM2-dependent ubiquitination of TP53 in response to radiation-induced DNA damage and during DNA replication. DCX(ERCC8) (the CSA complex) plays a role in transcription-coupled repair (TCR). May also play a role in ubiquitination of CDKN1B/p27kip when associated with CUL4 and SKP2.<ref>PMID:12732143</ref> <ref>PMID:15448697</ref> <ref>PMID:14739464</ref> <ref>PMID:15882621</ref> <ref>PMID:16260596</ref> <ref>PMID:16482215</ref> <ref>PMID:17079684</ref> <ref>PMID:16407242</ref> <ref>PMID:16407252</ref> <ref>PMID:16678110</ref> <ref>PMID:16940174</ref> <ref>PMID:17041588</ref> <ref>PMID:16473935</ref> <ref>PMID:18593899</ref> <ref>PMID:18381890</ref> <ref>PMID:18332868</ref> | [https://www.uniprot.org/uniprot/DDB1_HUMAN DDB1_HUMAN] Required for DNA repair. Binds to DDB2 to form the UV-damaged DNA-binding protein complex (the UV-DDB complex). The UV-DDB complex may recognize UV-induced DNA damage and recruit proteins of the nucleotide excision repair pathway (the NER pathway) to initiate DNA repair. The UV-DDB complex preferentially binds to cyclobutane pyrimidine dimers (CPD), 6-4 photoproducts (6-4 PP), apurinic sites and short mismatches. Also appears to function as a component of numerous distinct DCX (DDB1-CUL4-X-box) E3 ubiquitin-protein ligase complexes which mediate the ubiquitination and subsequent proteasomal degradation of target proteins. The functional specificity of the DCX E3 ubiquitin-protein ligase complex is determined by the variable substrate recognition component recruited by DDB1. DCX(DDB2) (also known as DDB1-CUL4-ROC1, CUL4-DDB-ROC1 and CUL4-DDB-RBX1) may ubiquitinate histone H2A, histone H3 and histone H4 at sites of UV-induced DNA damage. The ubiquitination of histones may facilitate their removal from the nucleosome and promote subsequent DNA repair. DCX(DDB2) also ubiquitinates XPC, which may enhance DNA-binding by XPC and promote NER. DCX(DTL) plays a role in PCNA-dependent polyubiquitination of CDT1 and MDM2-dependent ubiquitination of TP53 in response to radiation-induced DNA damage and during DNA replication. DCX(ERCC8) (the CSA complex) plays a role in transcription-coupled repair (TCR). May also play a role in ubiquitination of CDKN1B/p27kip when associated with CUL4 and SKP2.<ref>PMID:12732143</ref> <ref>PMID:15448697</ref> <ref>PMID:14739464</ref> <ref>PMID:15882621</ref> <ref>PMID:16260596</ref> <ref>PMID:16482215</ref> <ref>PMID:17079684</ref> <ref>PMID:16407242</ref> <ref>PMID:16407252</ref> <ref>PMID:16678110</ref> <ref>PMID:16940174</ref> <ref>PMID:17041588</ref> <ref>PMID:16473935</ref> <ref>PMID:18593899</ref> <ref>PMID:18381890</ref> <ref>PMID:18332868</ref> | ||
| - | <div style="background-color:#fffaf0;"> | ||
| - | == Publication Abstract from PubMed == | ||
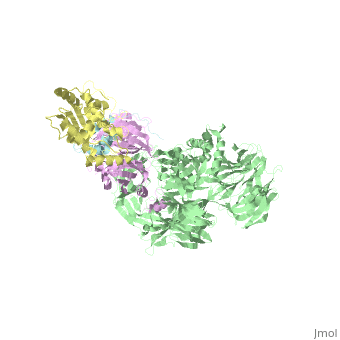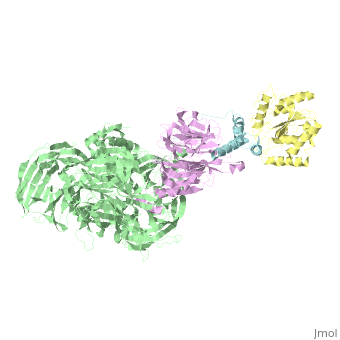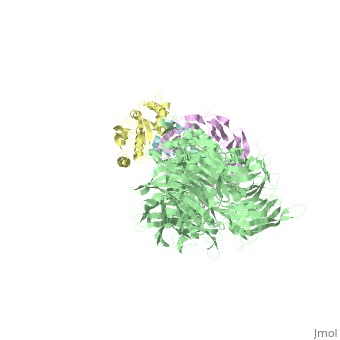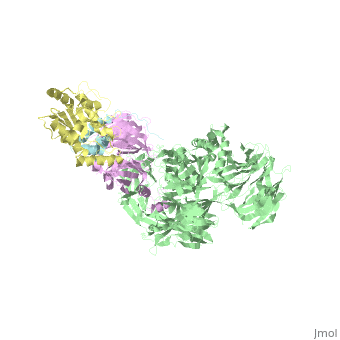
| - | The HIV-1 accessory protein Vpr is required for efficient viral infection of macrophages and promotion of viral replication in T cells. Vpr's biological activities are closely linked to the interaction with human DCAF1, a cellular substrate receptor of the Cullin4-RING E3 ubiquitin ligase (CRL4) of the host ubiquitin-proteasome-mediated protein degradation pathway. The molecular details of how Vpr usurps the protein degradation pathway have not been delineated. Here we present the crystal structure of the DDB1-DCAF1-HIV-1-Vpr-uracil-DNA glycosylase (UNG2) complex. The structure reveals how Vpr engages with DCAF1, creating a binding interface for UNG2 recruitment in a manner distinct from the recruitment of SAMHD1 by Vpx proteins. Vpr and Vpx use similar N-terminal and helical regions to bind the substrate receptor, whereas different regions target the specific cellular substrates. Furthermore, Vpr uses molecular mimicry of DNA by a variable loop for specific recruitment of the UNG2 substrate. | ||
| - | |||
| - | The DDB1-DCAF1-Vpr-UNG2 crystal structure reveals how HIV-1 Vpr steers human UNG2 toward destruction.,Wu Y, Zhou X, Barnes CO, DeLucia M, Cohen AE, Gronenborn AM, Ahn J, Calero G Nat Struct Mol Biol. 2016 Aug 29. doi: 10.1038/nsmb.3284. PMID:27571178<ref>PMID:27571178</ref> | ||
| - | |||
| - | From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | ||
| - | </div> | ||
| - | <div class="pdbe-citations 5jk7" style="background-color:#fffaf0;"></div> | ||
==See Also== | ==See Also== | ||
Current revision
The X-ray structure of the DDB1-DCAF1-Vpr-UNG2 complex
| |||||||||||
Categories: Homo sapiens | Large Structures | Ahn J | Calero G | Wu Y