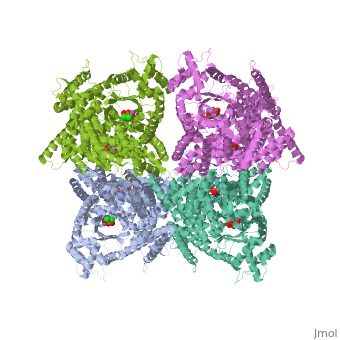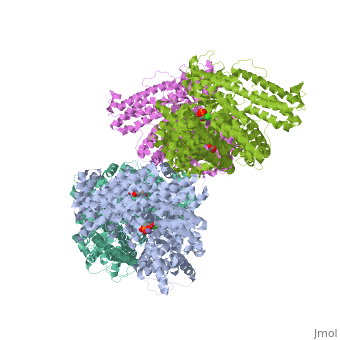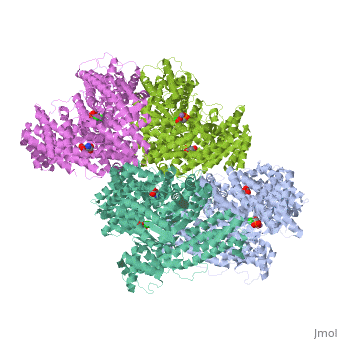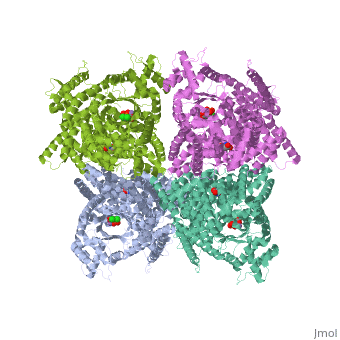
1jqn
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
<StructureSection load='1jqn' size='340' side='right'caption='[[1jqn]], [[Resolution|resolution]] 2.35Å' scene=''> | <StructureSection load='1jqn' size='340' side='right'caption='[[1jqn]], [[Resolution|resolution]] 2.35Å' scene=''> | ||
== Structural highlights == | == Structural highlights == | ||
| - | <table><tr><td colspan='2'>[[1jqn]] is a 1 chain structure with sequence from [ | + | <table><tr><td colspan='2'>[[1jqn]] is a 1 chain structure with sequence from [https://en.wikipedia.org/wiki/Escherichia_coli Escherichia coli]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1JQN OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=1JQN FirstGlance]. <br> |
| - | </td></tr><tr id=' | + | </td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 2.35Å</td></tr> |
| - | + | <tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=ASP:ASPARTIC+ACID'>ASP</scene>, <scene name='pdbligand=DCO:3,3-DICHLORO-2-PHOSPHONOMETHYL-ACRYLIC+ACID'>DCO</scene>, <scene name='pdbligand=MN:MANGANESE+(II)+ION'>MN</scene></td></tr> | |
| - | <tr id=' | + | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=1jqn FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1jqn OCA], [https://pdbe.org/1jqn PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=1jqn RCSB], [https://www.ebi.ac.uk/pdbsum/1jqn PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=1jqn ProSAT]</span></td></tr> |
| - | < | + | |
| - | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[ | + | |
</table> | </table> | ||
== Function == | == Function == | ||
| - | [ | + | [https://www.uniprot.org/uniprot/CAPP_ECOLI CAPP_ECOLI] Forms oxaloacetate, a four-carbon dicarboxylic acid source for the tricarboxylic acid cycle.[HAMAP-Rule:MF_00595] |
== Evolutionary Conservation == | == Evolutionary Conservation == | ||
[[Image:Consurf_key_small.gif|200px|right]] | [[Image:Consurf_key_small.gif|200px|right]] | ||
| Line 22: | Line 20: | ||
</jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=1jqn ConSurf]. | </jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=1jqn ConSurf]. | ||
<div style="clear:both"></div> | <div style="clear:both"></div> | ||
| - | <div style="background-color:#fffaf0;"> | ||
| - | == Publication Abstract from PubMed == | ||
| - | Phosphoenolpyruvate carboxylase (PEPC) catalyzes the first step in the fixation of atmospheric CO(2) during C(4) photosynthesis. The crystal structure of C(4) form maize PEPC (ZmPEPC), the first structure of the plant PEPCs, has been determined at 3.0 A resolution. The structure includes a sulfate ion at the plausible binding site of an allosteric activator, glucose 6-phosphate. The crystal structure of E. coli PEPC (EcPEPC) complexed with Mn(2+), phosphoenolpyruvate analog (3,3-dichloro-2-dihydroxyphosphinoylmethyl-2-propenoate), and an allosteric inhibitor, aspartate, has also been determined at 2.35 A resolution. Dynamic movements were found in the ZmPEPC structure, compared with the EcPEPC structure, around two loops near the active site. On the basis of these molecular structures, the mechanisms for the carboxylation reaction and for the allosteric regulation of PEPC are proposed. | ||
| - | |||
| - | Crystal structures of C4 form maize and quaternary complex of E. coli phosphoenolpyruvate carboxylases.,Matsumura H, Xie Y, Shirakata S, Inoue T, Yoshinaga T, Ueno Y, Izui K, Kai Y Structure. 2002 Dec;10(12):1721-30. PMID:12467579<ref>PMID:12467579</ref> | ||
| - | |||
| - | From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | ||
| - | </div> | ||
| - | <div class="pdbe-citations 1jqn" style="background-color:#fffaf0;"></div> | ||
==See Also== | ==See Also== | ||
*[[Phosphoenolpyruvate carboxylase|Phosphoenolpyruvate carboxylase]] | *[[Phosphoenolpyruvate carboxylase|Phosphoenolpyruvate carboxylase]] | ||
| - | == References == | ||
| - | <references/> | ||
__TOC__ | __TOC__ | ||
</StructureSection> | </StructureSection> | ||
| - | [[Category: | + | [[Category: Escherichia coli]] |
[[Category: Large Structures]] | [[Category: Large Structures]] | ||
| - | + | [[Category: Kai Y]] | |
| - | [[Category: Kai | + | [[Category: Matsumura H]] |
| - | [[Category: Matsumura | + | |
| - | + | ||
| - | + | ||
| - | + | ||
Current revision
Crystal structure of E.coli phosphoenolpyruvate carboxylase in complex with Mn2+ and DCDP
| |||||||||||