1azx
From Proteopedia
(Difference between revisions)
| Line 4: | Line 4: | ||
== Structural highlights == | == Structural highlights == | ||
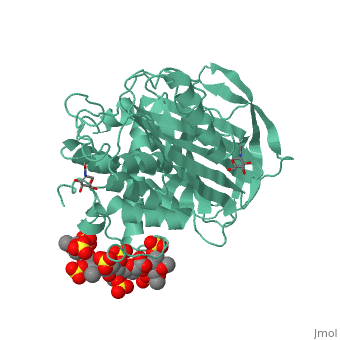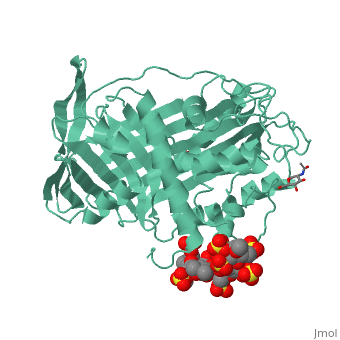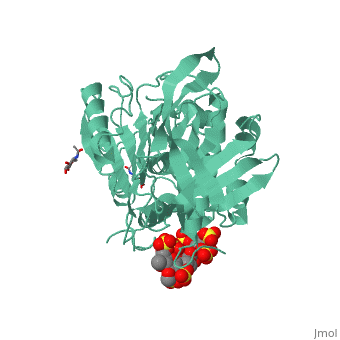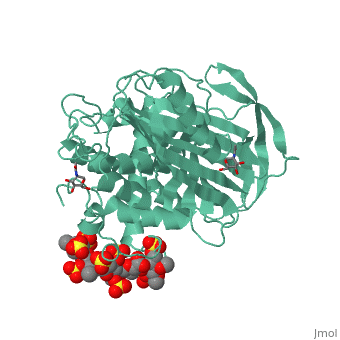
<table><tr><td colspan='2'>[[1azx]] is a 2 chain structure with sequence from [https://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1AZX OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=1AZX FirstGlance]. <br> | <table><tr><td colspan='2'>[[1azx]] is a 2 chain structure with sequence from [https://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1AZX OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=1AZX FirstGlance]. <br> | ||
| - | </td></tr><tr id=' | + | </td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 2.9Å</td></tr> |
| - | < | + | <tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=GU1:2,3-DI-O-METHYL-BETA-D-GLUCOPYRANURONIC+ACID'>GU1</scene>, <scene name='pdbligand=GU6:2,3,6-TRI-O-SULFONATO-ALPHA-D-GLUCOPYRANOSE'>GU6</scene>, <scene name='pdbligand=NAG:N-ACETYL-D-GLUCOSAMINE'>NAG</scene>, <scene name='pdbligand=PRD_900031:heparin+pentasaccharide'>PRD_900031</scene>, <scene name='pdbligand=Z9H:3,4-di-O-methyl-2,6-di-O-sulfo-alpha-D-glucopyranose'>Z9H</scene>, <scene name='pdbligand=Z9K:3-O-methyl-2-O-sulfo-alpha-L-idopyranuronic+acid'>Z9K</scene>, <scene name='pdbligand=Z9L:methyl+2,3,6-tri-O-sulfo-alpha-D-glucopyranoside'>Z9L</scene></td></tr> |
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=1azx FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1azx OCA], [https://pdbe.org/1azx PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=1azx RCSB], [https://www.ebi.ac.uk/pdbsum/1azx PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=1azx ProSAT]</span></td></tr> | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=1azx FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1azx OCA], [https://pdbe.org/1azx PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=1azx RCSB], [https://www.ebi.ac.uk/pdbsum/1azx PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=1azx ProSAT]</span></td></tr> | ||
</table> | </table> | ||
== Disease == | == Disease == | ||
| - | + | [https://www.uniprot.org/uniprot/ANT3_HUMAN ANT3_HUMAN] Defects in SERPINC1 are the cause of antithrombin III deficiency (AT3D) [MIM:[https://omim.org/entry/613118 613118]. AT3D is an important risk factor for hereditary thrombophilia, a hemostatic disorder characterized by a tendency to recurrent thrombosis. AT3D is classified into 4 types. Type I: characterized by a 50% decrease in antigenic and functional levels. Type II: has defects affecting the thrombin-binding domain. Type III: alteration of the heparin-binding domain. Plasma AT-III antigen levels are normal in type II and III. Type IV: consists of miscellaneous group of unclassifiable mutations.<ref>PMID:7734359</ref> [:]<ref>PMID:3191114</ref> <ref>PMID:9031473</ref> <ref>PMID:6582486</ref> <ref>PMID:3080419</ref> <ref>PMID:3805013</ref> <ref>PMID:3179438</ref> <ref>PMID:3162733</ref> <ref>PMID:2781509</ref> <ref>PMID:2365065</ref> <ref>PMID:2229057</ref> <ref>PMID:2013320</ref> <ref>PMID:1906811</ref> <ref>PMID:1555650</ref> <ref>PMID:1547341</ref> <ref>PMID:8443391</ref> <ref>PMID:8486379</ref> <ref>PMID:7981186</ref> <ref>PMID:7959685</ref> <ref>PMID:8274732</ref> <ref>PMID:7994035</ref> <ref>PMID:7989582</ref> [:]<ref>PMID:7878627</ref> <ref>PMID:7832187</ref> <ref>PMID:9157604</ref> <ref>PMID:9845533</ref> <ref>PMID:9759613</ref> <ref>PMID:10997988</ref> <ref>PMID:11794707</ref> <ref>PMID:11713457</ref> <ref>PMID:12353073</ref> <ref>PMID:12595305</ref> <ref>PMID:12894857</ref> <ref>PMID:15164384</ref> <ref>PMID:16908819</ref> | |
== Function == | == Function == | ||
| - | + | [https://www.uniprot.org/uniprot/ANT3_HUMAN ANT3_HUMAN] Most important serine protease inhibitor in plasma that regulates the blood coagulation cascade. AT-III inhibits thrombin, matriptase-3/TMPRSS7, as well as factors IXa, Xa and XIa. Its inhibitory activity is greatly enhanced in the presence of heparin.<ref>PMID:15853774</ref> | |
== Evolutionary Conservation == | == Evolutionary Conservation == | ||
[[Image:Consurf_key_small.gif|200px|right]] | [[Image:Consurf_key_small.gif|200px|right]] | ||
| Line 22: | Line 22: | ||
</jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=1azx ConSurf]. | </jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=1azx ConSurf]. | ||
<div style="clear:both"></div> | <div style="clear:both"></div> | ||
| - | <div style="background-color:#fffaf0;"> | ||
| - | == Publication Abstract from PubMed == | ||
| - | Antithrombin, a plasma serpin, is relatively inactive as an inhibitor of the coagulation proteases until it binds to the heparan side chains that line the microvasculature. The binding specifically occurs to a core pentasaccharide present both in the heparans and in their therapeutic derivative heparin. The accompanying conformational change of antithrombin is revealed in a 2.9-A structure of a dimer of latent and active antithrombins, each in complex with the high-affinity pentasaccharide. Inhibitory activation results from a shift in the main sheet of the molecule from a partially six-stranded to a five-stranded form, with extrusion of the reactive center loop to give a more exposed orientation. There is a tilting and elongation of helix D with the formation of a 2-turn helix P between the C and D helices. Concomitant conformational changes at the heparin binding site explain both the initial tight binding of antithrombin to the heparans and the subsequent release of the antithrombin-protease complex into the circulation. The pentasaccharide binds by hydrogen bonding of its sulfates and carboxylates to Arg-129 and Lys-125 in the D-helix, to Arg-46 and Arg-47 in the A-helix, to Lys-114 and Glu-113 in the P-helix, and to Lys-11 and Arg-13 in a cleft formed by the amino terminus. This clear definition of the binding site will provide a structural basis for developing heparin analogues that are more specific toward their intended target antithrombin and therefore less likely to exhibit side effects. | ||
| - | |||
| - | The anticoagulant activation of antithrombin by heparin.,Jin L, Abrahams JP, Skinner R, Petitou M, Pike RN, Carrell RW Proc Natl Acad Sci U S A. 1997 Dec 23;94(26):14683-8. PMID:9405673<ref>PMID:9405673</ref> | ||
| - | |||
| - | From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | ||
| - | </div> | ||
| - | <div class="pdbe-citations 1azx" style="background-color:#fffaf0;"></div> | ||
==See Also== | ==See Also== | ||
| Line 40: | Line 31: | ||
[[Category: Homo sapiens]] | [[Category: Homo sapiens]] | ||
[[Category: Large Structures]] | [[Category: Large Structures]] | ||
| - | [[Category: Abrahams | + | [[Category: Abrahams JP]] |
| - | [[Category: Carrell | + | [[Category: Carrell RW]] |
| - | [[Category: Jin | + | [[Category: Jin L]] |
| - | [[Category: Petitou | + | [[Category: Petitou M]] |
| - | [[Category: Pike | + | [[Category: Pike RN]] |
| - | [[Category: Skinner | + | [[Category: Skinner R]] |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
Revision as of 15:29, 13 March 2024
ANTITHROMBIN/PENTASACCHARIDE COMPLEX
| |||||||||||
Categories: Homo sapiens | Large Structures | Abrahams JP | Carrell RW | Jin L | Petitou M | Pike RN | Skinner R