User:Brynn Baker/Sandbox1
From Proteopedia
| Line 7: | Line 7: | ||
== Amylin == | == Amylin == | ||
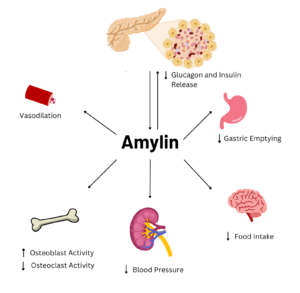
| - | Amylin is a neuroendocrine hormone that is synthesized with insulin in the [https://en.wikipedia.org/wiki/Beta_cell beta cells] of pancreatic islets. It can cross the [https://en.wikipedia.org/wiki/Blood%E2%80%93brain_barrier blood-brain barrier] and regulates glucose homeostasis via inhibiting gastric emptying, inhibiting the release of glucagon, and inducing meal-ending satiety<ref name="Hay">PMID:26071095</ref>. In doing so, it prevents spikes in blood glucose and overeating, making it a suitable target for [https://en.wikipedia.org/wiki/Type_2_diabetes Type 2 Diabetes] treatments and therapies. Seeing as Type 2 Diabetes is a major risk factor for [https://en.wikipedia.org/wiki/Alzheimer%27s_disease Alzheimer's Disease], as Type 2 Diabetes cases continue to increase, there will likely be a spike in Alzheimer’s Disease as well <ref name="Grizzanti">PMID:30282360</ref>. Therefore, it is vital that amylin, its receptor, and analogs, such as pramlintide, are understood to aid in rational drug design. | + | Amylin is a neuroendocrine hormone that is synthesized with insulin in the [https://en.wikipedia.org/wiki/Beta_cell beta cells] of pancreatic islets. It can cross the [https://en.wikipedia.org/wiki/Blood%E2%80%93brain_barrier blood-brain barrier] and regulates glucose homeostasis via inhibiting gastric emptying, inhibiting the release of glucagon, and inducing meal-ending satiety<ref name="Hay">PMID:26071095</ref>. In doing so, it prevents spikes in blood glucose and overeating, making it a suitable target for [https://en.wikipedia.org/wiki/Type_2_diabetes Type 2 Diabetes] treatments and therapies. Seeing as Type 2 Diabetes is a major risk factor for [https://en.wikipedia.org/wiki/Alzheimer%27s_disease Alzheimer's Disease], as Type 2 Diabetes cases continue to increase, there will likely be a spike in Alzheimer’s Disease as well<ref name="Grizzanti">PMID:30282360</ref>. Therefore, it is vital that amylin, its receptor, and analogs, such as pramlintide, are understood to aid in rational drug design. |
[[Image:AmylinFlowchart.png|300 px|left|thumb|Figure 2. Effects of Amylin in Humans]] | [[Image:AmylinFlowchart.png|300 px|left|thumb|Figure 2. Effects of Amylin in Humans]] | ||
=== Structure === | === Structure === | ||
== Amylin Receptor == | == Amylin Receptor == | ||
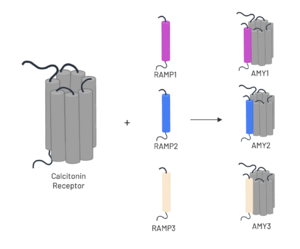
| - | The amylin receptor (AMYR) is the result of the heterodimerization of the [https://en.wikipedia.org/wiki/Calcitonin_receptor calcitonin receptor (CT)] and a [https://en.wikipedia.org/wiki/Receptor_activity-modifying_protein receptor-activity modifying protein (RAMP)]. The patterns of peptide interaction between CT and AMYR are very similar overall, but amylin has a higher affinity for AMYR1 and AMYR3 than AMYR2. | + | The amylin receptor (AMYR) is the result of the heterodimerization of the [https://en.wikipedia.org/wiki/Calcitonin_receptor calcitonin receptor (CT)] and a [https://en.wikipedia.org/wiki/Receptor_activity-modifying_protein receptor-activity modifying protein (RAMP)]. The patterns of peptide interaction between CT and AMYR are very similar overall, but amylin has a higher affinity for AMYR1 and AMYR3 than AMYR2<ref name="Cao">PMID:35324283</ref>. |
[[Image:AMYR.png|300 px|right|thumb|Figure 3. Heterodimerization of CT and RAMPs]] | [[Image:AMYR.png|300 px|right|thumb|Figure 3. Heterodimerization of CT and RAMPs]] | ||
| Line 29: | Line 29: | ||
== Disease == | == Disease == | ||
=== Pramlintide Analogue === | === Pramlintide Analogue === | ||
| - | The first human amylin analogue, <scene name='10/1037520/Pramlintide_overall/1'>pramlintide</scene>, was developed in 1995, and marked a significant advancement in the treatment of Type 2 Diabetes <ref name="Bower">PMID:27061187</ref>. As of 2024, it is the only FDA-approved drug for the treatment of Type 2 Diabetes using the AMYR as a target. Recent studies in rodent Alzheimer’s Disease models suggest that pramlintide reduces amyloid-beta plaques, making it a potential therapeutic target for Alzheimer’s Disease <ref name="Grizzanti">PMID:30282360</ref>. | + | The first human amylin analogue, <scene name='10/1037520/Pramlintide_overall/1'>pramlintide</scene>, was developed in 1995, and marked a significant advancement in the treatment of Type 2 Diabetes<ref name="Bower">PMID:27061187</ref>. As of 2024, it is the only FDA-approved drug for the treatment of Type 2 Diabetes using the AMYR as a target. Recent studies in rodent Alzheimer’s Disease models suggest that pramlintide reduces amyloid-beta plaques, making it a potential therapeutic target for Alzheimer’s Disease<ref name="Grizzanti">PMID:30282360</ref>. |
There is limited knowledge about how human amylin binds to the human AMYR, so many studies utilize rat amylin as the peptide for the human AMYR. Rat amylin and pramlintide showcase very similar structures and maintain a high sequence similarity. For instance, the N-term lysine residue is conserved between rat amylin and pramlintide, as well as human amylin <ref name="Cao">PMID:35324283</ref>, suggesting that the lysine is integral for binding to AMYR. The residue changes between the two include <scene name='10/1037520/Rat_pram_18/2'>R18 in rat amylin to H18 in pramlintide</scene>, <scene name='10/1038871/Rat_pram_19/1'>S19 in rat amylin to K19 in pramlintide</scene>, L23 in rat amylin to F23 in pramlintide, and V26 in rat amylin to I26 in pramlintide. While these residues differ, the overall properties of the various residues remain consistent, so many of the same interactions with AMYR are likely still able to form. | There is limited knowledge about how human amylin binds to the human AMYR, so many studies utilize rat amylin as the peptide for the human AMYR. Rat amylin and pramlintide showcase very similar structures and maintain a high sequence similarity. For instance, the N-term lysine residue is conserved between rat amylin and pramlintide, as well as human amylin <ref name="Cao">PMID:35324283</ref>, suggesting that the lysine is integral for binding to AMYR. The residue changes between the two include <scene name='10/1037520/Rat_pram_18/2'>R18 in rat amylin to H18 in pramlintide</scene>, <scene name='10/1038871/Rat_pram_19/1'>S19 in rat amylin to K19 in pramlintide</scene>, L23 in rat amylin to F23 in pramlintide, and V26 in rat amylin to I26 in pramlintide. While these residues differ, the overall properties of the various residues remain consistent, so many of the same interactions with AMYR are likely still able to form. | ||
Revision as of 00:34, 10 April 2024
Homo sapiens Amylin3 Receptor, AMYR3
| |||||||||||
References
- ↑ Hay DL, Chen S, Lutz TA, Parkes DG, Roth JD. Amylin: Pharmacology, Physiology, and Clinical Potential. Pharmacol Rev. 2015 Jul;67(3):564-600. PMID:26071095 doi:10.1124/pr.115.010629
- ↑ 2.0 2.1 Grizzanti J, Corrigan R, Casadesus G. Neuroprotective Effects of Amylin Analogues on Alzheimer's Disease Pathogenesis and Cognition. J Alzheimers Dis. 2018;66(1):11-23. PMID:30282360 doi:10.3233/JAD-180433
- ↑ 3.0 3.1 Cao J, Belousoff MJ, Liang YL, Johnson RM, Josephs TM, Fletcher MM, Christopoulos A, Hay DL, Danev R, Wootten D, Sexton PM. A structural basis for amylin receptor phenotype. Science. 2022 Mar 25;375(6587):eabm9609. PMID:35324283 doi:10.1126/science.abm9609
- ↑ Bower RL, Hay DL. Amylin structure-function relationships and receptor pharmacology: implications for amylin mimetic drug development. Br J Pharmacol. 2016 Jun;173(12):1883-98. PMID:27061187 doi:10.1111/bph.13496
Bower, R.; Hay, D. Amylin structure-function relationships and receptor pharmacology: implications for amylin mimetic drug development. British Journal of Pharmacology. 2016, 173, 1883-1898. [1].
Cao, J.; Belousoff, J.; Liang, Y.; Johnson, R.; Josephs, T.; Fletcher, M.; Christopoulus, A.; Hay, D.; Danev, R.; Wootten, D.; Sexton, P. A structural basis for amylin receptor phenotype. Science. 2022, 375. [2].
Grizzanti, J.; Corrigan, R.; Casadesus, G. Neuroprotective Effects of Amylin Analogues on Alzheimer’s Disease Pathogenesis and Cognition. Journal of Alzheimer’s Disease. 2019, 66, 11-23. [3].
Hay, D.; Chen, S.; Lutz, T.; Parkes, D.; Roth, J. Amylin: Pharmacology, Physiology, and Clinical Potential. Pharmacological Reviews. 2015, 67, 564-600. [4].
Student Contributors
- Brynn Baker
- Emily Berkman
- Sepp Hall