1lqh
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
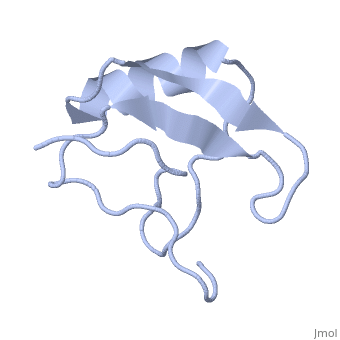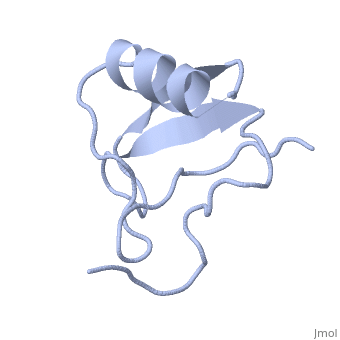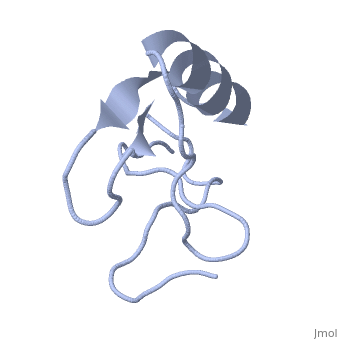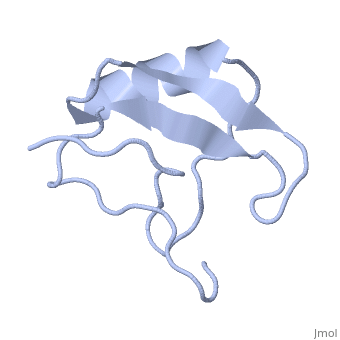
==INSECTICIDAL ALPHA SCORPION TOXIN ISOLATED FROM THE VENOM OF SCORPION LEIURUS QUINQUESTRIATUS HEBRAEUS, NMR, MINIMIZED AVERAGE STRUCTURE== | ==INSECTICIDAL ALPHA SCORPION TOXIN ISOLATED FROM THE VENOM OF SCORPION LEIURUS QUINQUESTRIATUS HEBRAEUS, NMR, MINIMIZED AVERAGE STRUCTURE== | ||
| - | <StructureSection load='1lqh' size='340' side='right'caption='[[1lqh | + | <StructureSection load='1lqh' size='340' side='right'caption='[[1lqh]]' scene=''> |
== Structural highlights == | == Structural highlights == | ||
| - | <table><tr><td colspan='2'>[[1lqh]] is a 1 chain structure with sequence from [https://en.wikipedia.org/wiki/ | + | <table><tr><td colspan='2'>[[1lqh]] is a 1 chain structure with sequence from [https://en.wikipedia.org/wiki/Leiurus_quinquestriatus_hebraeus Leiurus quinquestriatus hebraeus]. Full experimental information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1LQH OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=1LQH FirstGlance]. <br> |
| - | </td></tr><tr id=' | + | </td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">Solution NMR</td></tr> |
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=1lqh FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1lqh OCA], [https://pdbe.org/1lqh PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=1lqh RCSB], [https://www.ebi.ac.uk/pdbsum/1lqh PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=1lqh ProSAT]</span></td></tr> | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=1lqh FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1lqh OCA], [https://pdbe.org/1lqh PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=1lqh RCSB], [https://www.ebi.ac.uk/pdbsum/1lqh PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=1lqh ProSAT]</span></td></tr> | ||
</table> | </table> | ||
== Function == | == Function == | ||
| - | + | [https://www.uniprot.org/uniprot/SCXA_LEIHE SCXA_LEIHE] Alpha toxins bind voltage-independently at site-3 of sodium channels (Nav) and inhibit the inactivation of the activated channels, thereby blocking neuronal transmission. The dissociation is voltage-dependent. This toxin is active on insects. It is also highly toxic to crustaceans and has a measurable but low toxicity to mice. | |
== Evolutionary Conservation == | == Evolutionary Conservation == | ||
[[Image:Consurf_key_small.gif|200px|right]] | [[Image:Consurf_key_small.gif|200px|right]] | ||
| Line 19: | Line 19: | ||
</jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=1lqh ConSurf]. | </jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=1lqh ConSurf]. | ||
<div style="clear:both"></div> | <div style="clear:both"></div> | ||
| - | <div style="background-color:#fffaf0;"> | ||
| - | == Publication Abstract from PubMed == | ||
| - | The solution structure of a recombinant active alpha-neurotoxin from Leiurus quinquestriatus hebraeus, Lqh(alpha)IT, was determined by proton two-dimensional nuclear magnetic resonance spectroscopy (2D NMR). This toxin is the most insecticidal among scorpion alpha-neurotoxins and, therefore, serves as a model for clarifying the structural basis for their biological activity and selective toxicity. A set of 29 structures was generated without constraint violations exceeding 0.4 A. These structures had root mean square deviations of 0.49 and 1.00 A with respect to the average structure for backbone atoms and all heavy atoms, respectively. Similarly to other scorpion toxins, the structure of Lqh(alpha)IT consists of an alpha-helix, a three-strand antiparallel beta-sheet, three type I tight turns, a five-residue turn, and a hydrophobic patch that includes tyrosine and tryptophan rings in a "herringbone" arrangement. Positive phi angles were found for Ala50 and Asn11, suggesting their proximity to functionally important regions of the molecule. The sample exhibited conformational heterogeneity over a wide range of experimental conditions, and two conformations were observed for the majority of protein residues. The ratio between these conformations was temperature-dependent, and the rate of their interconversions was estimated to be on the order of 1-5 s(-1) at 308 K. The conformation of the polypeptide backbone of Lqh(alpha)IT is very similar to that of the most active antimammalian scorpion alpha-toxin, AaHII, from Androctonus australis Hector (60% amino acid sequence homology). Yet, several important differences were observed at the 5-residue turn comprising residues Lys8-Cys12, the C-terminal segment, and the mutual disposition of these two regions. 2D NMR studies of the R64H mutant, which is 3 times more toxic than the unmodified Lqh(alpha)IT, demonstrated the importance of the spatial orientation of the last residue side chain for toxicity of Lqh(alpha)IT. | ||
| - | |||
| - | Solution structures of a highly insecticidal recombinant scorpion alpha-toxin and a mutant with increased activity.,Tugarinov V, Kustanovich I, Zilberberg N, Gurevitz M, Anglister J Biochemistry. 1997 Mar 4;36(9):2414-24. PMID:9054546<ref>PMID:9054546</ref> | ||
| - | |||
| - | From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | ||
| - | </div> | ||
| - | <div class="pdbe-citations 1lqh" style="background-color:#fffaf0;"></div> | ||
==See Also== | ==See Also== | ||
| Line 33: | Line 24: | ||
*[[Potassium channel toxin|Potassium channel toxin]] | *[[Potassium channel toxin|Potassium channel toxin]] | ||
*[[Potassium channel toxin 3D structures|Potassium channel toxin 3D structures]] | *[[Potassium channel toxin 3D structures|Potassium channel toxin 3D structures]] | ||
| - | == References == | ||
| - | <references/> | ||
__TOC__ | __TOC__ | ||
</StructureSection> | </StructureSection> | ||
| - | [[Category: Deathstalker scorpion]] | ||
[[Category: Large Structures]] | [[Category: Large Structures]] | ||
| - | [[Category: Anglister | + | [[Category: Leiurus quinquestriatus hebraeus]] |
| - | [[Category: Gurevitz | + | [[Category: Anglister J]] |
| - | [[Category: Kustanovich | + | [[Category: Gurevitz M]] |
| - | [[Category: Tugarinov | + | [[Category: Kustanovich I]] |
| - | [[Category: Zilberberg | + | [[Category: Tugarinov V]] |
| - | + | [[Category: Zilberberg N]] | |
| - | + | ||
Revision as of 08:26, 10 April 2024
INSECTICIDAL ALPHA SCORPION TOXIN ISOLATED FROM THE VENOM OF SCORPION LEIURUS QUINQUESTRIATUS HEBRAEUS, NMR, MINIMIZED AVERAGE STRUCTURE
| |||||||||||