This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
User:SaraKathryn Kalkhoff/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
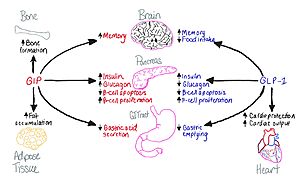
The GIP receptor helps facilitate movement of glucose within a cell. <ref name='Sun'>PMID:35333651</ref>. It has a natural ligand that is 42 residues and helps kickstart the GIP-R into firing, as a transporter for glucose in and out of the cell. Once the levels become too high, the ligand will send this transporter back down into the cell and not have anymore glucose activate. It will also cause the insulin pathway to start to help signal that there is too much glucose in the body. | The GIP receptor helps facilitate movement of glucose within a cell. <ref name='Sun'>PMID:35333651</ref>. It has a natural ligand that is 42 residues and helps kickstart the GIP-R into firing, as a transporter for glucose in and out of the cell. Once the levels become too high, the ligand will send this transporter back down into the cell and not have anymore glucose activate. It will also cause the insulin pathway to start to help signal that there is too much glucose in the body. | ||
===Tirzepatide=== | ===Tirzepatide=== | ||
| - | Tirzepatide has been used as a treatment for Type 2 diabetes and <scene name='10/1037492/Just_tirz/1'>Tirzepatide has a total of 39 residues present in its structure.</scene> It is used to help treat Type 2 Diabetes, as an agonist to allow insulin to be broken down. This also works in tandem with GLP-1 | + | Tirzepatide has been used as a treatment for Type 2 diabetes and <scene name='10/1037492/Just_tirz/1'>Tirzepatide has a total of 39 residues present in its structure.</scene> It is used to help treat Type 2 Diabetes, as an agonist to allow insulin to be broken down. This also works in tandem with GLP-1, as mentioned within Figure 1 earlier on. It also contains two residues with an AIB sequence, which stands for alpha-amino isobutric acid and aids with preventing degradation of the peptide <ref name='Chavda>PMID: 35807558</ref>. This sequence is part of the reason as to why the |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
== Structural highlights == | == Structural highlights == | ||
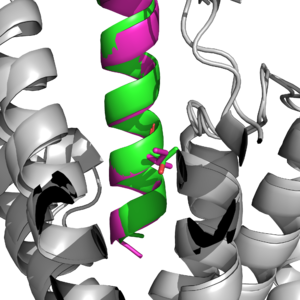
| - | <scene name='10/1037492/Gip_tirz/7'>Main scene of Tirzepatide bound to the GIP receptor. </scene> | + | <scene name='10/1037492/Gip_tirz/7'>Main scene of Tirzepatide bound to the GIP receptor. </scene> Tirzepatide and the natural GIP for the receptor contain some key differences, but they are all able to bind with a strong affinity to the receptor. |
=== Active Site === | === Active Site === | ||
Main binding domains between tirzepatide and the GIP receptor would contain an arginine 190 and glutamine 220 residues to facilitate binding of the ligand. The binding is also able to use a tyrosine residue at position 1 that helps guide the ligand into the correct binding spot <author here>.One key difference found was a point mutation at position 7 between an Isoleucine and Threonine <ref name='Sun'>PMID:35333651</ref>, which would result in a higher affinity for the tirzepatide molecule binding onto the receptor than the ligand. | Main binding domains between tirzepatide and the GIP receptor would contain an arginine 190 and glutamine 220 residues to facilitate binding of the ligand. The binding is also able to use a tyrosine residue at position 1 that helps guide the ligand into the correct binding spot <author here>.One key difference found was a point mutation at position 7 between an Isoleucine and Threonine <ref name='Sun'>PMID:35333651</ref>, which would result in a higher affinity for the tirzepatide molecule binding onto the receptor than the ligand. | ||
Revision as of 02:25, 13 April 2024
| |||||||||||