We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Ben Whiteside
From Proteopedia
(Difference between revisions)
| Line 19: | Line 19: | ||
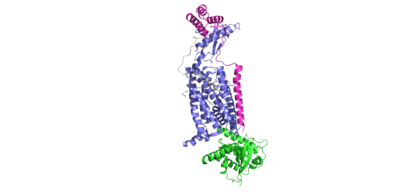
<scene name='10/1038828/Ramp_ctr_interface/9'>RAMP CTR Interface </scene> is a key interaction that stabilizes the protein complex and positions the receptor to favorably bind to amylin. The RAMP-CTR interface extends into the plasma membrane, providing additional non-covalent bonding between the protein complex and the cell membrane. | <scene name='10/1038828/Ramp_ctr_interface/9'>RAMP CTR Interface </scene> is a key interaction that stabilizes the protein complex and positions the receptor to favorably bind to amylin. The RAMP-CTR interface extends into the plasma membrane, providing additional non-covalent bonding between the protein complex and the cell membrane. | ||
==== Extracellular Domain - RAMP interactions ==== | ==== Extracellular Domain - RAMP interactions ==== | ||
| - | <scene name='10/1038828/Ctr_ramp_ecd_stablization/7'>RAMP CTR Extracellular Domain Interaction</scene> | + | The extracellular domain of the CTR primarily contains polar residues in the extracellular space. In order to orient these residues in such a way to facilitate amylin binding, RAMP makes hydrogen bonds with the CTR to increase the rigidity of the receptor binding site. <scene name='10/1038828/Ctr_ramp_ecd_stablization/7'>RAMP CTR Extracellular Domain Interaction</scene> |
=== Bypass Motif === | === Bypass Motif === | ||
=== G-alpha Interactions with CTR TMD === | === G-alpha Interactions with CTR TMD === | ||
| - | <scene name='10/1038828/Ctr_g_alpha/13'> | + | In order to transduce the signal across the cell membrane, the binding of amylin will induce a conformational change that allows for the CTR to make favorable interactions with the G alpha subunit. Two interactions shown <scene name='10/1038828/Ctr_g_alpha/13'>here </scene> and <scene name='10/1038828/Ctr_g_alpha/12'>here </scene> activate the G-protein and propel downstream signaling. |
| - | <scene name='10/1038828/Ctr_g_alpha/12'> | + | |
== Function == | == Function == | ||
Revision as of 14:42, 16 April 2024
AMYR
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ Ransey E, Paredes E, Dey SK, Das SR, Heroux A, Macbeth MR. Crystal structure of the Entamoeba histolytica RNA lariat debranching enzyme EhDbr1 reveals a catalytic Zn(2+) /Mn(2+) heterobinucleation. FEBS Lett. 2017 Jul;591(13):2003-2010. doi: 10.1002/1873-3468.12677. Epub 2017, Jun 14. PMID:28504306 doi:http://dx.doi.org/10.1002/1873-3468.12677
- ↑ Cao J, Belousoff MJ, Liang YL, Johnson RM, Josephs TM, Fletcher MM, Christopoulos A, Hay DL, Danev R, Wootten D, Sexton PM. A structural basis for amylin receptor phenotype. Science. 2022 Mar 25;375(6587):eabm9609. PMID:35324283 doi:10.1126/science.abm9609
Student Contributors
Ben Whiteside, Mathias Vander Eide, Andrew Helmerich,