User:Karisma Moll/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
[https://www.sciencedirect.com/science/article/pii/S0304416520301483#t0005 Proline Peptidase Overview] | [https://www.sciencedirect.com/science/article/pii/S0304416520301483#t0005 Proline Peptidase Overview] | ||
=== History === | === History === | ||
| - | Dipeptidyl Peptidase IV's role in the inactivation of [https://en.wikipedia.org/wiki/Incretin incretin hormones] was discovered in the 1990s. Animal studies were conducted in the late 1990s, followed by human studies in the early 2000s. The first DPPIV inhibitors (sitagliptin, vildagliptin, alogliptin, saxagliptin, and linagliptin) were approved starting in 2006, and now serve as monotherapy or add-on to other therapies in a glucose-lowering capacity. <ref name="Ahrén">PMID:31275243</ref> Since their approval, there have been multiple long-term trials to continue exploring the long-term effects of these medications. It is known that gliptins directly impact the pancreas, kidney, heart, and vessels. The most investigated thus far is the effects of gliptins on | + | Dipeptidyl Peptidase IV's role in the inactivation of [https://en.wikipedia.org/wiki/Incretin incretin hormones] was discovered in the 1990s. Animal studies were conducted in the late 1990s, followed by human studies in the early 2000s. The first DPPIV inhibitors (sitagliptin, vildagliptin, alogliptin, saxagliptin, and linagliptin) were approved starting in 2006, and now serve as monotherapy or add-on to other therapies in a glucose-lowering capacity. <ref name="Ahrén">PMID:31275243</ref> Since their approval, there have been multiple long-term trials to continue exploring the long-term effects of these medications. It is known that gliptins directly impact the pancreas, kidney, heart, and vessels. The most investigated thus far is the effects of gliptins on renal and cardiovascular functioning. <ref name="Khalse">PMID:30294582</ref> Results of phase II and III trails indicated that gliptins did not harm the cardiovascular system. A meta-analysis implicated two possible beneficial effects for patients treated with these medications: a reduction in cardiovascular effects and a direct renoprotective effect. <ref name="Hocher">PMID:22947920</ref> |
=== Function === | === Function === | ||
| Line 29: | Line 29: | ||
| - | DPPIV is found in two forms in the body: a membrane bound monomer and a blood soluble dimer. All structural renderings of DPPIV start at the 39th residue, meaning it does not include the intracellular domain, transmembrane region, and part of the cleavage site. The monomer has 4 domains: {{font color|dimgray|DPPIV cleavage stalk}}, {{font color|tomato|beta propeller}}, {{font color|khaki|cystine-rich region}}, and the {{font color|mediumseagreen|catalytic domain}}. The DPPIV [https://en.wikipedia.org/wiki/Beta-propeller beta | + | DPPIV is found in two forms in the body: a membrane bound monomer and a blood soluble dimer. All structural renderings of DPPIV start at the 39th residue, meaning it does not include the intracellular domain, transmembrane region, and part of the cleavage site. The monomer has 4 domains: {{font color|dimgray|DPPIV cleavage stalk}}, {{font color|tomato|beta propeller}}, {{font color|khaki|cystine-rich region}}, and the {{font color|mediumseagreen|catalytic domain}}. |
| + | |||
| + | The DPPIV [https://en.wikipedia.org/wiki/Beta-propeller beta propeller] is notable as it differs from all the other enzymes in the [https://en.wikipedia.org/wiki/Dipeptidyl_peptidase dipeptidyl peptidase family]. In all other DPPs the beta propeller has ligand gating potential; however, the <scene name='10/1037489/Beta_propeller/2'>beta propeller</scene> is an asymmetrical 7 blade propeller that does not function as a ligand gate by rather acts as a binding site which allows DPPIV to conjugate with [https://en.wikipedia.org/wiki/Adenosine_deaminase Adenosine Deaminase]. <ref name="Abbott">PMID:10583373</ref> | ||
The <scene name='10/1037489/Cystine_rich_region/1'>cystine rich region</scene> contains 6 cystine residues (C385, C394, C444, C447, C454, C472) that make <scene name='10/1037489/Disulfide_bonds/1'>three disulfide bonds</scene> that play a critical role in the tertiary structure and therefore function of the DPPIV enzyme. <ref name="Dobers">PMID:10931192</ref> | The <scene name='10/1037489/Cystine_rich_region/1'>cystine rich region</scene> contains 6 cystine residues (C385, C394, C444, C447, C454, C472) that make <scene name='10/1037489/Disulfide_bonds/1'>three disulfide bonds</scene> that play a critical role in the tertiary structure and therefore function of the DPPIV enzyme. <ref name="Dobers">PMID:10931192</ref> | ||
| Line 36: | Line 38: | ||
=== Catalytic Triad === | === Catalytic Triad === | ||
| - | <scene name='10/1037493/Oxyanion_hole/1'>oxyanion hole</scene> | ||
<ref name="Hiramatsu"/> | <ref name="Hiramatsu"/> | ||
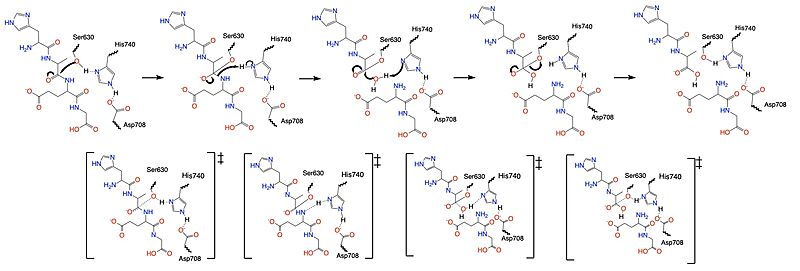
=== Mechanism === | === Mechanism === | ||
Revision as of 18:34, 17 April 2024
Structure and Function of Dipeptidyl Peptidase IV (DPPIV) in Humans
| |||||||||||
References
- ↑ Ahrén B. DPP-4 Inhibition and the Path to Clinical Proof. Front Endocrinol (Lausanne). 2019 Jun 19;10:376. PMID:31275243 doi:10.3389/fendo.2019.00376
- ↑ Khalse M, Bhargava A. A Review on Cardiovascular Outcome Studies of Dipeptidyl Peptidase-4 Inhibitors. Indian J Endocrinol Metab. 2018 Sep-Oct;22(5):689-695. PMID:30294582 doi:10.4103/ijem.IJEM_104_18
- ↑ Hocher B, Reichetzeder C, Alter ML. Renal and cardiac effects of DPP4 inhibitors--from preclinical development to clinical research. Kidney Blood Press Res. 2012;36(1):65-84. PMID:22947920 doi:10.1159/000339028
- ↑ Zhong J, Rajagopalan S. Dipeptidyl Peptidase-4 Regulation of SDF-1/CXCR4 Axis: Implications for Cardiovascular Disease. Front Immunol. 2015 Sep 25;6:477. PMID:26441982 doi:10.3389/fimmu.2015.00477
- ↑ Sharma A, Ren X, Zhang H, Pandey GN. Effect of depression and suicidal behavior on neuropeptide Y (NPY) and its receptors in the adult human brain: A postmortem study. Prog Neuropsychopharmacol Biol Psychiatry. 2022 Jan 10;112:110428. PMID:34411658 doi:10.1016/j.pnpbp.2021.110428
- ↑ Ntafam CN, Beutler BD, Harris RD. Incarcerated gravid uterus: A rare but potentially devastating obstetric complication. Radiol Case Rep. 2022 Mar 10;17(5):1583-1586. PMID:35309386 doi:10.1016/j.radcr.2022.02.034
- ↑ 7.0 7.1 Hiramatsu H, Kyono K, Higashiyama Y, Fukushima C, Shima H, Sugiyama S, Inaka K, Yamamoto A, Shimizu R. The structure and function of human dipeptidyl peptidase IV, possessing a unique eight-bladed beta-propeller fold. Biochem Biophys Res Commun. 2003 Mar 21;302(4):849-54. PMID:12646248
- ↑ Abbott CA, McCaughan GW, Levy MT, Church WB, Gorrell MD. Binding to human dipeptidyl peptidase IV by adenosine deaminase and antibodies that inhibit ligand binding involves overlapping, discontinuous sites on a predicted beta propeller domain. Eur J Biochem. 1999 Dec;266(3):798-810. PMID:10583373 doi:10.1046/j.1432-1327.1999.00902.x
- ↑ Dobers J, Grams S, Reutter W, Fan H. Roles of cysteines in rat dipeptidyl peptidase IV/CD26 in processing and proteolytic activity. Eur J Biochem. 2000 Aug;267(16):5093-100. PMID:10931192 doi:10.1046/j.1432-1327.2000.01571.x
- ↑ Kim BR, Kim HY, Choi I, Kim JB, Jin CH, Han AR. DPP-IV Inhibitory Potentials of Flavonol Glycosides Isolated from the Seeds of Lens culinaris: In Vitro and Molecular Docking Analyses. Molecules. 2018 Aug 10;23(8):1998. PMID:30103438 doi:10.3390/molecules23081998
Student Contributors
- Karisma Moll
- Merritt Jugo
- Sam Magnabosco