This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
User:Brynn Baker/Sandbox1
From Proteopedia
(Difference between revisions)
| Line 44: | Line 44: | ||
=== Pramlintide Analogue === | === Pramlintide Analogue === | ||
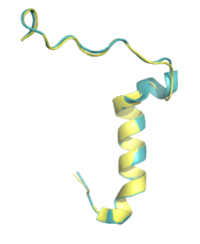
[[Image:Superimposed_april_15_2.png|200 px|right|thumb|Figure 4. Pramlintide (teal) and Rat Amylin (yellow) have very similar structures and binding interactions with AMYR3.]] | [[Image:Superimposed_april_15_2.png|200 px|right|thumb|Figure 4. Pramlintide (teal) and Rat Amylin (yellow) have very similar structures and binding interactions with AMYR3.]] | ||
| - | The first human amylin analogue, <scene name='10/1037520/Pramlintide_overall/ | + | The first human amylin analogue, <scene name='10/1037520/Pramlintide_overall/2'>pramlintide</scene>, was developed in 1995, and marked a significant advancement in the treatment of Type 2 Diabetes<ref name="Bower">PMID:27061187</ref>. As of 2024, it is the only FDA-approved drug for the treatment of Type 2 Diabetes using the AMYR as a target. Recent studies in rodent Alzheimer’s Disease models suggest that pramlintide reduces amyloid-beta plaques, making it a potential therapeutic target for Alzheimer’s Disease<ref name="Grizzanti">PMID:30282360</ref>. |
There is limited knowledge about how human amylin binds to the human AMYR, so many studies utilize rat amylin as the peptide for the human AMYR. Rat amylin and pramlintide showcase very similar structures and maintain a high sequence similarity. For instance, the N-term lysine residue is conserved between <scene name='10/1037520/Rat_k1/1'>rat amylin</scene> and <scene name='10/1037520/Pramlintide_k1/1'>pramlintide</scene>, as well as human amylin<ref name="Cao">PMID:35324283</ref>, suggesting that the lysine is integral for binding to AMYR. The residue changes between the two include <scene name='10/1037520/Rat_pram_18/2'>R18 in rat amylin to H18 in pramlintide</scene>, <scene name='10/1038871/Rat_pram_19/1'>S19 in rat amylin to K19 in pramlintide</scene>, <scene name='10/1037520/Rat_l23/2'>L23</scene> in rat amylin to <scene name='10/1037520/Pramlintide_f23/2'>F23</scene> in pramlintide, and <scene name='10/1037520/Rat_v26/3'>V26</scene> in rat amylin to <scene name='10/1037520/Pramlintide_i26/2'>I26</scene> in pramlintide. While these residues differ, the overall properties of the various residues remain consistent, so many of the same interactions with AMYR are likely still able to form. | There is limited knowledge about how human amylin binds to the human AMYR, so many studies utilize rat amylin as the peptide for the human AMYR. Rat amylin and pramlintide showcase very similar structures and maintain a high sequence similarity. For instance, the N-term lysine residue is conserved between <scene name='10/1037520/Rat_k1/1'>rat amylin</scene> and <scene name='10/1037520/Pramlintide_k1/1'>pramlintide</scene>, as well as human amylin<ref name="Cao">PMID:35324283</ref>, suggesting that the lysine is integral for binding to AMYR. The residue changes between the two include <scene name='10/1037520/Rat_pram_18/2'>R18 in rat amylin to H18 in pramlintide</scene>, <scene name='10/1038871/Rat_pram_19/1'>S19 in rat amylin to K19 in pramlintide</scene>, <scene name='10/1037520/Rat_l23/2'>L23</scene> in rat amylin to <scene name='10/1037520/Pramlintide_f23/2'>F23</scene> in pramlintide, and <scene name='10/1037520/Rat_v26/3'>V26</scene> in rat amylin to <scene name='10/1037520/Pramlintide_i26/2'>I26</scene> in pramlintide. While these residues differ, the overall properties of the various residues remain consistent, so many of the same interactions with AMYR are likely still able to form. | ||
Revision as of 21:11, 21 April 2024
Homo sapiens Amylin3 Receptor, AMYR3
| |||||||||||
References
- ↑ Hay DL, Chen S, Lutz TA, Parkes DG, Roth JD. Amylin: Pharmacology, Physiology, and Clinical Potential. Pharmacol Rev. 2015 Jul;67(3):564-600. PMID:26071095 doi:10.1124/pr.115.010629
- ↑ 2.0 2.1 Grizzanti J, Corrigan R, Casadesus G. Neuroprotective Effects of Amylin Analogues on Alzheimer's Disease Pathogenesis and Cognition. J Alzheimers Dis. 2018;66(1):11-23. PMID:30282360 doi:10.3233/JAD-180433
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 3.9 Cao J, Belousoff MJ, Liang YL, Johnson RM, Josephs TM, Fletcher MM, Christopoulos A, Hay DL, Danev R, Wootten D, Sexton PM. A structural basis for amylin receptor phenotype. Science. 2022 Mar 25;375(6587):eabm9609. PMID:35324283 doi:10.1126/science.abm9609
- ↑ Bower RL, Hay DL. Amylin structure-function relationships and receptor pharmacology: implications for amylin mimetic drug development. Br J Pharmacol. 2016 Jun;173(12):1883-98. PMID:27061187 doi:10.1111/bph.13496
Student Contributors
- Brynn Baker
- Emily Berkman
- Sepp Hall