User:Jaelin Lunato/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 12: | Line 12: | ||
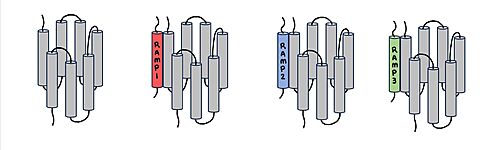
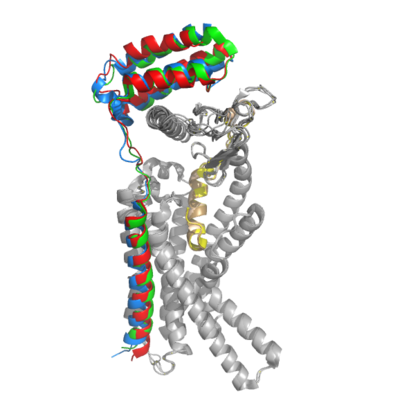
[[Image:RAMP_diagram.jpg|500px|right|thumb|'''Figure 1.''' Three different RAMPs to compose AMY1R, AMY2R, and AMY3R when associated with the calcitonin receptor (shown in grey). RAMP1 is in red, RAMP2 is in blue, and RAMP3 is in green.]] AMYR is a heterodimer of a calcitonin receptor (CTR) and a receptor activity-modifying protein (RAMP). There are three different RAMPs, RAMP1, RAMP2, and RAMP3, that compose AMY1R, AMY2R, and AMY3R when associated with the CTR (Figure 1). The three different RAMPs are structurally similar to each other, so all three RAMPs are able to bind to the CTR without any modification of the CTR (Figure 2). [[Image:Overlay of RAMPs.png|400 px|right|thumb|'''Figure 2.''' Superimposition of RAMP1, RAMP2, and RAMP3. RAMP1 is red, RAMP2 is blue, and RAMP3 is green. The amylin ligand is dark yellow, and the calcitonin ligand is pale yellow.]] | [[Image:RAMP_diagram.jpg|500px|right|thumb|'''Figure 1.''' Three different RAMPs to compose AMY1R, AMY2R, and AMY3R when associated with the calcitonin receptor (shown in grey). RAMP1 is in red, RAMP2 is in blue, and RAMP3 is in green.]] AMYR is a heterodimer of a calcitonin receptor (CTR) and a receptor activity-modifying protein (RAMP). There are three different RAMPs, RAMP1, RAMP2, and RAMP3, that compose AMY1R, AMY2R, and AMY3R when associated with the CTR (Figure 1). The three different RAMPs are structurally similar to each other, so all three RAMPs are able to bind to the CTR without any modification of the CTR (Figure 2). [[Image:Overlay of RAMPs.png|400 px|right|thumb|'''Figure 2.''' Superimposition of RAMP1, RAMP2, and RAMP3. RAMP1 is red, RAMP2 is blue, and RAMP3 is green. The amylin ligand is dark yellow, and the calcitonin ligand is pale yellow.]] | ||
| - | The two major ligands of the calcitonin receptor are [https://en.wikipedia.org/wiki/Calcitonin calcitonin] and [https://en.wikipedia.org/wiki/Amylin amylin]. In the absence of a RAMP, the calcitonin receptor has greater affinity for calcitonin than amylin, but because the <scene name='10/1037496/Overlay_of_ligands/2'>two ligands are structurally similar</scene>, both calcitonin and amylin can bind to the CTR without any modification of the receptor. When the CTR is bound to a RAMP, the complex becomes the AMYR and has greater affinity for the <scene name='10/1037495/Ligand_in_membrane/1'>amylin ligand</scene> relative to the calcitonin ligand.(REFERENCE NEEDED HERE******) | + | The two major ligands of the calcitonin receptor are [https://en.wikipedia.org/wiki/Calcitonin calcitonin] and [https://en.wikipedia.org/wiki/Amylin amylin]. In the absence of a RAMP, the calcitonin receptor has greater affinity for calcitonin than amylin, but because the <scene name='10/1037496/Overlay_of_ligands/2'>two ligands are structurally similar</scene>, both calcitonin and amylin can bind to the CTR without any modification of the receptor (Figure 3). When the CTR is bound to a RAMP, the complex becomes the AMYR and has greater affinity for the <scene name='10/1037495/Ligand_in_membrane/1'>amylin ligand</scene> relative to the calcitonin ligand.(REFERENCE NEEDED HERE******)[[Image:Sequence alignment ligands.png|500px|left|thumb|'''Figure 3.''' Sequence alignment of rat amylin and salmon calcitonin. Conserved residues are highlighted in blue.]] |
| - | + | ||
| - | [[Image:Sequence alignment ligands.png|500px|left|thumb|Sequence alignment of rat amylin and salmon calcitonin.]] | + | |
| - | + | ||
There are two required post-translational modifications of amylin in order for the ligand to have any bioactivity: (1) <scene name='10/1037495/C-term_amide/1'>amidation of the C-terminus</scene> and (2) a <scene name='10/1037495/Amylin_disulfide_bond2/4'>disulfide bond</scene> between C2 and C7. | There are two required post-translational modifications of amylin in order for the ligand to have any bioactivity: (1) <scene name='10/1037495/C-term_amide/1'>amidation of the C-terminus</scene> and (2) a <scene name='10/1037495/Amylin_disulfide_bond2/4'>disulfide bond</scene> between C2 and C7. | ||
| Line 22: | Line 19: | ||
There are water molecules present in the binding site between amylin and the calcitonin receptor that support the ligand-receptor interaction. Some water molecules interact with the amylin ligand and create water-bridged Hydrogen bonds between different ligand residues, such as the <scene name='10/1037495/Water_1_ver3/4'>water-bridged Hydrogen bond between the main chains of T6 and T9</scene>. Other water molecules create <scene name='10/1037495/Water_receptor/2'>water-bridged Hydrogen bonds between residues of the calcitonin receptor</scene>. The water molecules are present in the empty space located in the ligand binding site, and they are hypothesized to stabilize the active conformation of the calcitonin receptor when amylin is bound. Substitutions of polar residues involved with the water-bridged Hydrogen bond network to nonpolar residues causes a decrease in potency and affinity of amylin to the calcitonin receptor. (REFERENCE NEEDED HERE******) | There are water molecules present in the binding site between amylin and the calcitonin receptor that support the ligand-receptor interaction. Some water molecules interact with the amylin ligand and create water-bridged Hydrogen bonds between different ligand residues, such as the <scene name='10/1037495/Water_1_ver3/4'>water-bridged Hydrogen bond between the main chains of T6 and T9</scene>. Other water molecules create <scene name='10/1037495/Water_receptor/2'>water-bridged Hydrogen bonds between residues of the calcitonin receptor</scene>. The water molecules are present in the empty space located in the ligand binding site, and they are hypothesized to stabilize the active conformation of the calcitonin receptor when amylin is bound. Substitutions of polar residues involved with the water-bridged Hydrogen bond network to nonpolar residues causes a decrease in potency and affinity of amylin to the calcitonin receptor. (REFERENCE NEEDED HERE******) | ||
| - | |||
There are <scene name='10/1037495/Amylin_2hbonds/5'>two conserved hydrogen bonds between the CTR and the amylin N-terminus loop</scene>. These bonds contribute to the functional phenotype of AMYR and also causes the end of the amylin ligand to be held in a flipped up position. | There are <scene name='10/1037495/Amylin_2hbonds/5'>two conserved hydrogen bonds between the CTR and the amylin N-terminus loop</scene>. These bonds contribute to the functional phenotype of AMYR and also causes the end of the amylin ligand to be held in a flipped up position. | ||
| Line 37: | Line 33: | ||
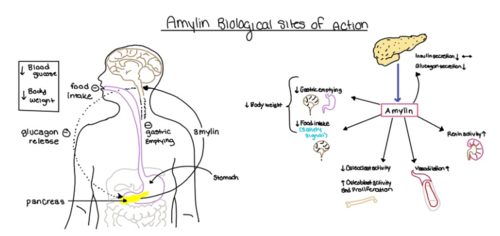
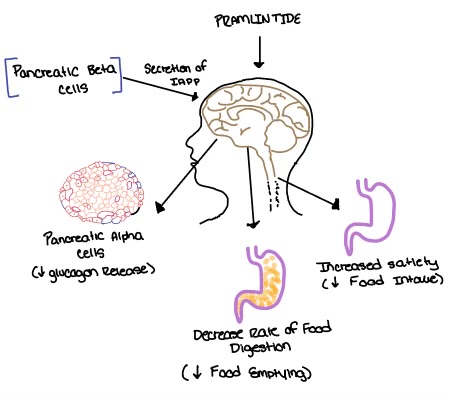
| - | [[Image:Amylin_biological_role.png| | + | [[Image:Amylin_biological_role.png|500 px|right|thumb|'''Figure 4.''' Biological role of amylin.]] |
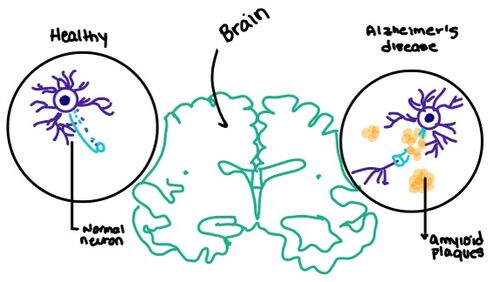
[[Image:Amylin brain.jpeg|500 px|right|thumb|Figure number]] | [[Image:Amylin brain.jpeg|500 px|right|thumb|Figure number]] | ||
Revision as of 21:46, 22 April 2024
Amylin Receptor (AMYR)
| |||||||||||