Structure
Cellular Domains
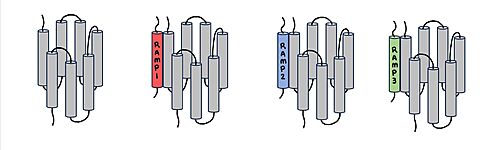
Figure 1. Three different RAMPs to compose AMY1R, AMY2R, and AMY3R when associated with the calcitonin receptor (shown in grey). RAMP1 is in red, RAMP2 is in blue, and RAMP3 is in green.
The amylin receptor, AMYR, has three domains: extracellular, transmembrane, and intracellular. The
calcitonin receptor, CTR, and the
receptor activity-modifying protein, RAMP, have both and . The ligand, amylin, binds within the transmembrane domain. The
G protein is located . The location of each component of the amylin receptor is essential in determining structure and function.
Receptor components
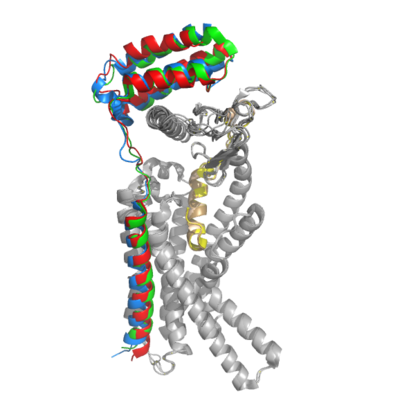
Figure 2. Superimposition of RAMP1, RAMP2, and RAMP3. RAMP1 is red, RAMP2 is blue, and RAMP3 is green. The amylin ligand is dark yellow, and the calcitonin ligand is pale yellow.
AMYR is a heterodimer of a calcitonin receptor (CTR) and a receptor activity-modifying protein (RAMP). There are three different RAMPs, RAMP1, RAMP2, and RAMP3, that compose AMY1R, AMY2R, and AMY3R when associated with the CTR (Figure 1). The three different RAMPs are structurally similar to each other, so all three RAMPs are able to bind to the CTR without any modification of the CTR (Figure 2).
The two major ligands of the calcitonin receptor are
calcitonin and
amylin. In the absence of a RAMP, the calcitonin receptor has greater affinity for calcitonin than amylin, but because the , both calcitonin and amylin can bind to the CTR without any modification of the receptor (Figure 3). When the CTR is bound to a RAMP, the complex becomes the AMYR and has greater affinity for the relative to the calcitonin ligand.(REFERENCE NEEDED HERE******)

Figure 3. Sequence alignment of rat amylin and salmon calcitonin. Conserved residues are highlighted in blue.
There are two required post-translational modifications of amylin in order for the ligand to have any bioactivity: (1) and (2) a between C2 and C7.
Binding Site Residues
There are water molecules present in the binding site between amylin and the calcitonin receptor that support the ligand-receptor interaction. Some water molecules interact with the amylin ligand and create water-bridged Hydrogen bonds between different ligand residues, such as the . Other water molecules create . The water molecules are present in the empty space located in the ligand binding site, and they are hypothesized to stabilize the active conformation of the calcitonin receptor when amylin is bound. Substitutions of polar residues involved with the water-bridged Hydrogen bond network to nonpolar residues causes a decrease in potency and affinity of amylin to the calcitonin receptor. (REFERENCE NEEDED HERE******)
There are . These bonds contribute to the functional phenotype of AMYR and also causes the end of the amylin ligand to be held in a flipped up position.
G Protein Activation
There are extensive nonpolar, . Here, Val322 and Leu323 of the CTR 2nd intracellular loop makes nonpolar interactions with Ile248 and Val249 back to itself as well as Leu388 of the Gα subunit. The . The 3rd intracellular loop has Arg180 hydrogen bonding to the Gln384 of the Gα subunit.
Function
Biological Relevance
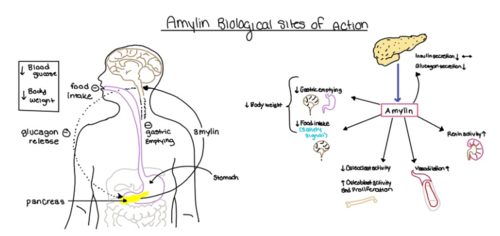
Figure 4. Different effects of amylin in the human body.
The functional pharmacology of AMYRs has relied on interference from differences between the behavior of CTRs in the presence and absence of RAMPs. Thus, understanding the structural basis for binding and selectivity of peptides to CTR and AMYRs is important for future drug discovery and development.
Diabetes
Amylin, as it is a part of the calcitonin peptide family, is heavily related to the regulation of homeostatic processes to relevant drug targets. Amylin is the target for the treatment of diabetes. Amylin is a neuroendocrine hormone that is synthesized and co-secreted with insulin. Insulin triggers glucose uptake which removes glucose from the bloodstream using it then for energy. Amylin works in negatively regulating (inhibiting) the formation of glucagon so that glucose polymers can continue to be broken down into the bloodstream for further energy storage and consumption. Therefore with the co-secretion of both amylin and insulin, it would aid in decreasing blood glucose levels thus becoming a predominant treatment plan for diabetic disorders, such as shown with the developing drug Pramlintide.
Pramlintide

Figure 5. Sequence alignment of rat amylin and pramlintide.
Pramlintide, a peptide analog of human amylin, is FDA-approved for the treatment of insulin-requiring diabetes (Figure 5). Pramlintide is injected into the bloodstream by the beta cells of the pancreas along with insulin after a meal, aiding in the regulation of blood glucose by slowing gastric emptying, promoting satiety via hypothalamic receptors, and inhibiting secretion of glucagon which opposes the effects of insulin and amylin (Figure 6).
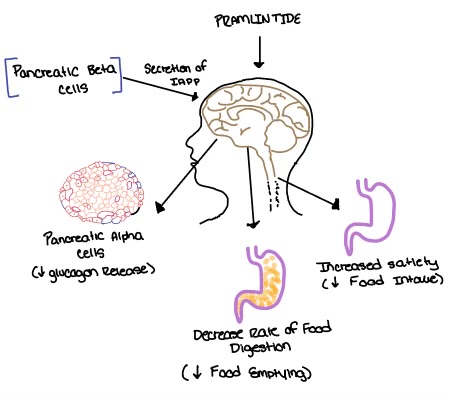
Figure 6. Pramlintide's effect on the human body. Pramlintide is a peptide agonist of human amylin and is an FDA approved treatment for diabetes.
Alzheimer's
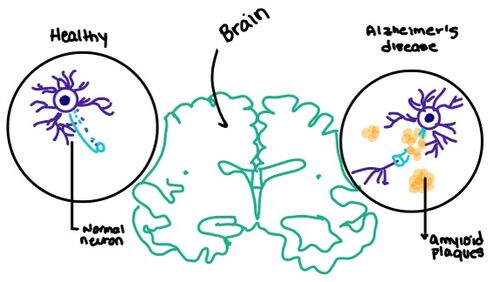
Figure 7. Amylin's effect on the brain through the buildup of amyloid plaques.
Alzheimer's is a neurodegenerative disease that progressively worsens due to an abundance of amyloid plaques building up in the grey matter of the brain. Amyloid plaques, also known as neuritic plaques or senile plaques, are extracellular deposits of the amyloid beta protein within the brain region. Abnormal levels of amylin-containing plaques clump together to create deposits within the aging brain to disrupt cell function. Understanding this disruption is important given the structural overlap of both the Amylin and Calcitonin binding sites in the brain.







