User:Karisma Moll/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 25: | Line 25: | ||
<scene name='10/1037489/Sax_intxn/1'>sax intxn</scene> | <scene name='10/1037489/Sax_intxn/1'>sax intxn</scene> | ||
<scene name='10/1037489/Vildagliptin_intxns/3'>new vild</scene> | <scene name='10/1037489/Vildagliptin_intxns/3'>new vild</scene> | ||
| - | + | <scene name='10/1037489/Sax_bonding/2'>scene caption sax bonding</scene> | |
| + | <scene name='10/1037489/Sax_surface/2'>scene caption sax surface</scene> | ||
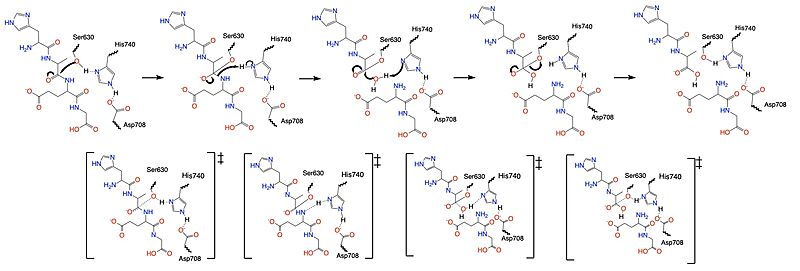
DPPIV is found in two forms in the body: a membrane bound monomer and a <scene name='10/1037489/Homodimer/1'>blood soluble homodimer</scene>. All structural renderings of DPPIV start at the 39th residue, meaning it does not include the intracellular domain, transmembrane region, and part of the cleavage site. The monomer has 4 domains: {{font color|dimgray|DPPIV cleavage stalk}}, {{font color|tomato|beta propeller}}, {{font color|khaki|cystine-rich region}}, and the {{font color|mediumseagreen|catalytic domain}}. | DPPIV is found in two forms in the body: a membrane bound monomer and a <scene name='10/1037489/Homodimer/1'>blood soluble homodimer</scene>. All structural renderings of DPPIV start at the 39th residue, meaning it does not include the intracellular domain, transmembrane region, and part of the cleavage site. The monomer has 4 domains: {{font color|dimgray|DPPIV cleavage stalk}}, {{font color|tomato|beta propeller}}, {{font color|khaki|cystine-rich region}}, and the {{font color|mediumseagreen|catalytic domain}}. | ||
Revision as of 13:17, 23 April 2024
Structure and Function of Dipeptidyl Peptidase IV (DPPIV) in Humans
| |||||||||||
References
- ↑ Ahrén B. DPP-4 Inhibition and the Path to Clinical Proof. Front Endocrinol (Lausanne). 2019 Jun 19;10:376. PMID:31275243 doi:10.3389/fendo.2019.00376
- ↑ Khalse M, Bhargava A. A Review on Cardiovascular Outcome Studies of Dipeptidyl Peptidase-4 Inhibitors. Indian J Endocrinol Metab. 2018 Sep-Oct;22(5):689-695. PMID:30294582 doi:10.4103/ijem.IJEM_104_18
- ↑ Hocher B, Reichetzeder C, Alter ML. Renal and cardiac effects of DPP4 inhibitors--from preclinical development to clinical research. Kidney Blood Press Res. 2012;36(1):65-84. PMID:22947920 doi:10.1159/000339028
- ↑ Zhong J, Rajagopalan S. Dipeptidyl Peptidase-4 Regulation of SDF-1/CXCR4 Axis: Implications for Cardiovascular Disease. Front Immunol. 2015 Sep 25;6:477. PMID:26441982 doi:10.3389/fimmu.2015.00477
- ↑ Sharma A, Ren X, Zhang H, Pandey GN. Effect of depression and suicidal behavior on neuropeptide Y (NPY) and its receptors in the adult human brain: A postmortem study. Prog Neuropsychopharmacol Biol Psychiatry. 2022 Jan 10;112:110428. PMID:34411658 doi:10.1016/j.pnpbp.2021.110428
- ↑ Ntafam CN, Beutler BD, Harris RD. Incarcerated gravid uterus: A rare but potentially devastating obstetric complication. Radiol Case Rep. 2022 Mar 10;17(5):1583-1586. PMID:35309386 doi:10.1016/j.radcr.2022.02.034
- ↑ 7.0 7.1 Hiramatsu H, Kyono K, Higashiyama Y, Fukushima C, Shima H, Sugiyama S, Inaka K, Yamamoto A, Shimizu R. The structure and function of human dipeptidyl peptidase IV, possessing a unique eight-bladed beta-propeller fold. Biochem Biophys Res Commun. 2003 Mar 21;302(4):849-54. PMID:12646248
- ↑ Abbott CA, McCaughan GW, Levy MT, Church WB, Gorrell MD. Binding to human dipeptidyl peptidase IV by adenosine deaminase and antibodies that inhibit ligand binding involves overlapping, discontinuous sites on a predicted beta propeller domain. Eur J Biochem. 1999 Dec;266(3):798-810. PMID:10583373 doi:10.1046/j.1432-1327.1999.00902.x
- ↑ Dobers J, Grams S, Reutter W, Fan H. Roles of cysteines in rat dipeptidyl peptidase IV/CD26 in processing and proteolytic activity. Eur J Biochem. 2000 Aug;267(16):5093-100. PMID:10931192 doi:10.1046/j.1432-1327.2000.01571.x
- ↑ Kim BR, Kim HY, Choi I, Kim JB, Jin CH, Han AR. DPP-IV Inhibitory Potentials of Flavonol Glycosides Isolated from the Seeds of Lens culinaris: In Vitro and Molecular Docking Analyses. Molecules. 2018 Aug 10;23(8):1998. PMID:30103438 doi:10.3390/molecules23081998
- ↑ Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007 Oct;87(4):1409-39. PMID:17928588 doi:10.1152/physrev.00034.2006
- ↑ Mulvihill EE, Drucker DJ. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr Rev. 2014 Dec;35(6):992-1019. PMID:25216328 doi:10.1210/er.2014-1035
Student Contributors
- Karisma Moll
- Merritt Jugo
- Sam Magnabosco