We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Karisma Moll/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 38: | Line 38: | ||
<ref name="Hiramatsu"/> | <ref name="Hiramatsu"/> | ||
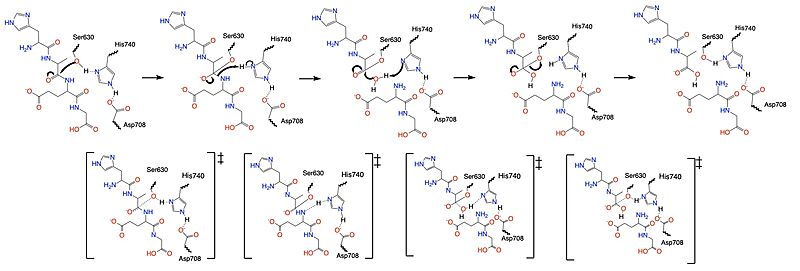
=== Mechanism === | === Mechanism === | ||
| - | <scene name='10/1037489/Glp1_peptide__scissile_bond/ | + | <scene name='10/1037489/Glp1_peptide__scissile_bond/2'>scene caption scissile bond</scene> |
Once a substrate is bound in the active site, DPPIV utilizes a [https://en.wikipedia.org/wiki/Enzyme_catalysis#Covalent_catalysis covalent catalysis] mechanism to cleave the substrate at the penultimate position. Asp708 of the <scene name='10/1037489/Catalytic_triad/7'>catalytic triad</scene> (Ser630, His 740, Asp708) pulls electron density from His740, allowing the histidine to pull electron density from Ser630, making serine a stronger nucleophile. The catalytic triad is assisted in this process by the oxyanion hole (residue Y631), providing stability and keeping the substrate in place. The water molecule attacks the carbonyl carbon, breaking the newly formed covalent bond, and releasing the first two residues of the starting substrate. The active site resets. | Once a substrate is bound in the active site, DPPIV utilizes a [https://en.wikipedia.org/wiki/Enzyme_catalysis#Covalent_catalysis covalent catalysis] mechanism to cleave the substrate at the penultimate position. Asp708 of the <scene name='10/1037489/Catalytic_triad/7'>catalytic triad</scene> (Ser630, His 740, Asp708) pulls electron density from His740, allowing the histidine to pull electron density from Ser630, making serine a stronger nucleophile. The catalytic triad is assisted in this process by the oxyanion hole (residue Y631), providing stability and keeping the substrate in place. The water molecule attacks the carbonyl carbon, breaking the newly formed covalent bond, and releasing the first two residues of the starting substrate. The active site resets. | ||
Revision as of 13:34, 23 April 2024
Structure and Function of Dipeptidyl Peptidase IV (DPPIV) in Humans
| |||||||||||
References
- ↑ Ahrén B. DPP-4 Inhibition and the Path to Clinical Proof. Front Endocrinol (Lausanne). 2019 Jun 19;10:376. PMID:31275243 doi:10.3389/fendo.2019.00376
- ↑ Khalse M, Bhargava A. A Review on Cardiovascular Outcome Studies of Dipeptidyl Peptidase-4 Inhibitors. Indian J Endocrinol Metab. 2018 Sep-Oct;22(5):689-695. PMID:30294582 doi:10.4103/ijem.IJEM_104_18
- ↑ Hocher B, Reichetzeder C, Alter ML. Renal and cardiac effects of DPP4 inhibitors--from preclinical development to clinical research. Kidney Blood Press Res. 2012;36(1):65-84. PMID:22947920 doi:10.1159/000339028
- ↑ Zhong J, Rajagopalan S. Dipeptidyl Peptidase-4 Regulation of SDF-1/CXCR4 Axis: Implications for Cardiovascular Disease. Front Immunol. 2015 Sep 25;6:477. PMID:26441982 doi:10.3389/fimmu.2015.00477
- ↑ Sharma A, Ren X, Zhang H, Pandey GN. Effect of depression and suicidal behavior on neuropeptide Y (NPY) and its receptors in the adult human brain: A postmortem study. Prog Neuropsychopharmacol Biol Psychiatry. 2022 Jan 10;112:110428. PMID:34411658 doi:10.1016/j.pnpbp.2021.110428
- ↑ Ntafam CN, Beutler BD, Harris RD. Incarcerated gravid uterus: A rare but potentially devastating obstetric complication. Radiol Case Rep. 2022 Mar 10;17(5):1583-1586. PMID:35309386 doi:10.1016/j.radcr.2022.02.034
- ↑ 7.0 7.1 Hiramatsu H, Kyono K, Higashiyama Y, Fukushima C, Shima H, Sugiyama S, Inaka K, Yamamoto A, Shimizu R. The structure and function of human dipeptidyl peptidase IV, possessing a unique eight-bladed beta-propeller fold. Biochem Biophys Res Commun. 2003 Mar 21;302(4):849-54. PMID:12646248
- ↑ Abbott CA, McCaughan GW, Levy MT, Church WB, Gorrell MD. Binding to human dipeptidyl peptidase IV by adenosine deaminase and antibodies that inhibit ligand binding involves overlapping, discontinuous sites on a predicted beta propeller domain. Eur J Biochem. 1999 Dec;266(3):798-810. PMID:10583373 doi:10.1046/j.1432-1327.1999.00902.x
- ↑ Dobers J, Grams S, Reutter W, Fan H. Roles of cysteines in rat dipeptidyl peptidase IV/CD26 in processing and proteolytic activity. Eur J Biochem. 2000 Aug;267(16):5093-100. PMID:10931192 doi:10.1046/j.1432-1327.2000.01571.x
- ↑ Kim BR, Kim HY, Choi I, Kim JB, Jin CH, Han AR. DPP-IV Inhibitory Potentials of Flavonol Glycosides Isolated from the Seeds of Lens culinaris: In Vitro and Molecular Docking Analyses. Molecules. 2018 Aug 10;23(8):1998. PMID:30103438 doi:10.3390/molecules23081998
- ↑ Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007 Oct;87(4):1409-39. PMID:17928588 doi:10.1152/physrev.00034.2006
- ↑ Mulvihill EE, Drucker DJ. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr Rev. 2014 Dec;35(6):992-1019. PMID:25216328 doi:10.1210/er.2014-1035
Student Contributors
- Karisma Moll
- Merritt Jugo
- Sam Magnabosco