User:Karisma Moll/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 50: | Line 50: | ||
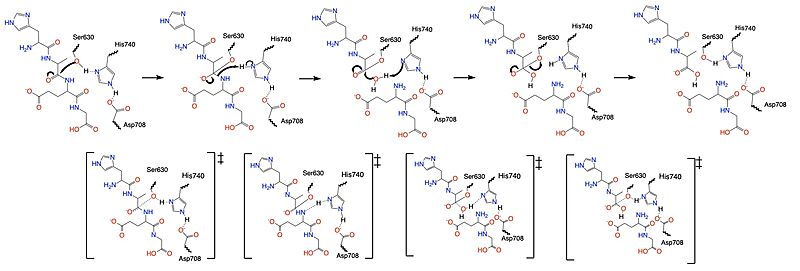
DPP-IV binds and degrades GLP-1 <scene name='10/1037489/Glp1_peptide__scissile_bond/3'>(cleaving at the penultimate position)</scene> resulting in heightened blood glucose levels. Insufficient GLP-1 production or signaling in response to meal intake is clinically associated with T2DM and morbidity. Since GLP-1 is a potent regulator of blood glucose levels, and DPP-IV antagonistically regulates GLP-1, this makes DPP-IV inhibitors an excellent candidate for pharmacological therapeutics for T2DM. <ref name="Gilbert">PMID:32308645</ref> | DPP-IV binds and degrades GLP-1 <scene name='10/1037489/Glp1_peptide__scissile_bond/3'>(cleaving at the penultimate position)</scene> resulting in heightened blood glucose levels. Insufficient GLP-1 production or signaling in response to meal intake is clinically associated with T2DM and morbidity. Since GLP-1 is a potent regulator of blood glucose levels, and DPP-IV antagonistically regulates GLP-1, this makes DPP-IV inhibitors an excellent candidate for pharmacological therapeutics for T2DM. <ref name="Gilbert">PMID:32308645</ref> | ||
| - | |||
| - | |||
| - | is a 30 amino acid hormone which is secreted into the gut by intestinal epithelial L-cells.<ref name="Holst">PMID:17928588</ref> GLP-1 is secreted in response to meal intake and is responsible for stimulating insulin production. GLP-1 is a major peptide which regulates blood glucose levels and is also a major contributor to DPPIV substrates. The concentration of active GLP-1 is tightly regulated by DPPIV. GLP-1 is rapidly metabolized and degraded by DPPIV before even reaching the gut, which results in increased blood glucose levels. Insufficient GLP production or signaling in response to meal intake as been clinically associated with [https://en.wikipedia.org/wiki/Type_2_diabetes Type 2 diabetes] and morbidity given the close [https://en.wikipedia.org/wiki/Receptor_antagonist antagonist correlation] between GLP production and blood glucose levels. The antagonistic regulation of GLP-1 by DPP-IV has made DPP-IV inhibition a very excellent candidate for pharmacological therapeutics. <ref name="Mulvihill">PMID:25216328</ref> | ||
| - | |||
Revision as of 14:49, 23 April 2024
Structure and Function of Dipeptidyl Peptidase IV (DPP-IV) in Humans
| |||||||||||
References
- ↑ Ahrén B. DPP-4 Inhibition and the Path to Clinical Proof. Front Endocrinol (Lausanne). 2019 Jun 19;10:376. PMID:31275243 doi:10.3389/fendo.2019.00376
- ↑ Khalse M, Bhargava A. A Review on Cardiovascular Outcome Studies of Dipeptidyl Peptidase-4 Inhibitors. Indian J Endocrinol Metab. 2018 Sep-Oct;22(5):689-695. PMID:30294582 doi:10.4103/ijem.IJEM_104_18
- ↑ Hocher B, Reichetzeder C, Alter ML. Renal and cardiac effects of DPP4 inhibitors--from preclinical development to clinical research. Kidney Blood Press Res. 2012;36(1):65-84. PMID:22947920 doi:10.1159/000339028
- ↑ Zhong J, Rajagopalan S. Dipeptidyl Peptidase-4 Regulation of SDF-1/CXCR4 Axis: Implications for Cardiovascular Disease. Front Immunol. 2015 Sep 25;6:477. PMID:26441982 doi:10.3389/fimmu.2015.00477
- ↑ Sharma A, Ren X, Zhang H, Pandey GN. Effect of depression and suicidal behavior on neuropeptide Y (NPY) and its receptors in the adult human brain: A postmortem study. Prog Neuropsychopharmacol Biol Psychiatry. 2022 Jan 10;112:110428. PMID:34411658 doi:10.1016/j.pnpbp.2021.110428
- ↑ Ntafam CN, Beutler BD, Harris RD. Incarcerated gravid uterus: A rare but potentially devastating obstetric complication. Radiol Case Rep. 2022 Mar 10;17(5):1583-1586. PMID:35309386 doi:10.1016/j.radcr.2022.02.034
- ↑ Abbott CA, McCaughan GW, Levy MT, Church WB, Gorrell MD. Binding to human dipeptidyl peptidase IV by adenosine deaminase and antibodies that inhibit ligand binding involves overlapping, discontinuous sites on a predicted beta propeller domain. Eur J Biochem. 1999 Dec;266(3):798-810. PMID:10583373 doi:10.1046/j.1432-1327.1999.00902.x
- ↑ Dobers J, Grams S, Reutter W, Fan H. Roles of cysteines in rat dipeptidyl peptidase IV/CD26 in processing and proteolytic activity. Eur J Biochem. 2000 Aug;267(16):5093-100. PMID:10931192 doi:10.1046/j.1432-1327.2000.01571.x
- ↑ Kim BR, Kim HY, Choi I, Kim JB, Jin CH, Han AR. DPP-IV Inhibitory Potentials of Flavonol Glycosides Isolated from the Seeds of Lens culinaris: In Vitro and Molecular Docking Analyses. Molecules. 2018 Aug 10;23(8):1998. PMID:30103438 doi:10.3390/molecules23081998
- ↑ Hiramatsu H, Kyono K, Higashiyama Y, Fukushima C, Shima H, Sugiyama S, Inaka K, Yamamoto A, Shimizu R. The structure and function of human dipeptidyl peptidase IV, possessing a unique eight-bladed beta-propeller fold. Biochem Biophys Res Commun. 2003 Mar 21;302(4):849-54. PMID:12646248
- ↑ Banday MZ, Sameer AS, Nissar S. Pathophysiology of diabetes: An overview. Avicenna J Med. 2020 Oct 13;10(4):174-188. PMID:33437689 doi:10.4103/ajm.ajm_53_20
- ↑ Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007 Oct;87(4):1409-39. PMID:17928588 doi:10.1152/physrev.00034.2006
- ↑ Gilbert MP, Pratley RE. GLP-1 Analogs and DPP-4 Inhibitors in Type 2 Diabetes Therapy: Review of Head-to-Head Clinical Trials. Front Endocrinol (Lausanne). 2020 Apr 3;11:178. PMID:32308645 doi:10.3389/fendo.2020.00178
Student Contributors
- Karisma Moll
- Merritt Jugo
- Sam Magnabosco