User:Jaelin Lunato/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 9: | Line 9: | ||
== Structure == | == Structure == | ||
=== Cellular Domains === | === Cellular Domains === | ||
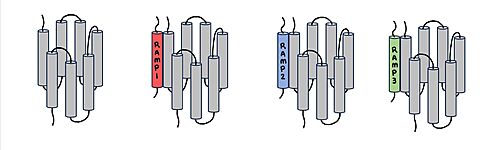
| - | [[Image:RAMP_diagram.jpg|500px|right|thumb|'''Figure 1.''' Three different RAMPs to compose AMY1R, AMY2R, and AMY3R when associated with the calcitonin receptor (shown in grey). RAMP1 is in red, RAMP2 is in blue, and RAMP3 is in green.]] AMYR has three domains: extracellular, transmembrane, and intracellular. The CTR and the RAMP have both <scene name='10/1037495/Extracellular_domain/7'>extracellular domains</scene> and <scene name='10/1037495/Transmembrane/6'>transmembrane domains</scene>. The calcitonin receptor is a 7-pass helix chain to which RAMP binds to become the amylin receptor. The ligand, amylin, binds within the transmembrane domain. The [https://en.wikipedia.org/wiki/G_protein G protein] is located <scene name='10/1037495/Intracellular_domain/2'>inside the cell</scene>. The location of each component of the amylin receptor is essential in determining structure and function. For example, it is important for the G protein to be located inside the cell in order to initiate a signal cascade for cell signaling. | + | [[Image:RAMP_diagram.jpg|500px|right|thumb|'''Figure 1.''' Three different RAMPs to compose AMY1R, AMY2R, and AMY3R when associated with the calcitonin receptor (shown in grey). RAMP1 is in red, RAMP2 is in blue, and RAMP3 is in green.]] AMYR has three domains: extracellular, transmembrane, and intracellular. The CTR and the RAMP have both <scene name='10/1037495/Extracellular_domain/7'>extracellular domains</scene> and <scene name='10/1037495/Transmembrane/6'>transmembrane domains</scene>. The calcitonin receptor is a 7-pass helix chain to which RAMP binds to become the amylin receptor <ref name="Hay">PMID:26071095</ref>. The ligand, amylin, binds within the transmembrane domain. The [https://en.wikipedia.org/wiki/G_protein G protein] is located <scene name='10/1037495/Intracellular_domain/2'>inside the cell</scene>. The location of each component of the amylin receptor is essential in determining structure and function. For example, it is important for the G protein to be located inside the cell in order to initiate a signal cascade for cell signaling. |
=== CTR and RAMP Heterodimer === | === CTR and RAMP Heterodimer === | ||
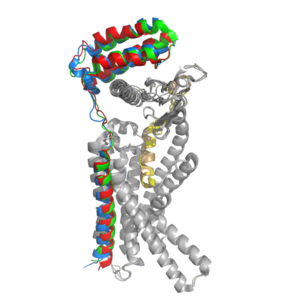
| - | [[Image:Overlay of RAMPs.png| | + | [[Image:Overlay of RAMPs.png|300 px|right|thumb|'''Figure 2.''' Superimposition of RAMP1, RAMP2, and RAMP3. RAMP1 is red, RAMP2 is blue, and RAMP3 is green. The amylin ligand is dark yellow, and the calcitonin ligand is pale yellow.]] AMYR is a heterodimer of a calcitonin receptor and a receptor activity-modifying protein. There are three different RAMPs, RAMP1, RAMP2, and RAMP3, that compose AMY1R, AMY2R, and AMY3R when associated with the CTR (Figure 1). The three different RAMPs are structurally similar to each other, so all three RAMPs are able to bind to the CTR without any modification of the CTR (Figure 2) <ref name="Hay">PMID:26071095</ref>. |
| - | In the absence of a RAMP, the calcitonin receptor has greater affinity for calcitonin than amylin, but because the <scene name='10/1037495/Overlay_of_ligands/1'>two ligands are structurally similar</scene>, both calcitonin and amylin can bind to the CTR without any modification of the receptor. The two ligands have many conserved residues (highlighted in blue), and this shows that the two ligands share many chemical properties which explains why they are structurally similar. In addition to having many conserved residues, both ligands also have an amidated C-terminus (Figure 3). When the CTR is bound to a RAMP, the complex becomes the AMYR and has greater affinity for the <scene name='10/1037495/Ligand_in_membrane/2'>amylin ligand</scene> relative to the calcitonin ligand. Therefore, the RAMP is essential to AMYR because it causes AMYR to have greater affinity for the amylin ligand rather than the calcitonin ligand. [[Image:Sequence alignment ligands.png|500px|left|thumb|'''Figure 3.''' Sequence alignment of rat amylin and salmon calcitonin. Conserved residues are highlighted in blue.]] | + | In the absence of a RAMP, the calcitonin receptor has greater affinity for calcitonin than amylin, but because the <scene name='10/1037495/Overlay_of_ligands/1'>two ligands are structurally similar</scene>, both calcitonin and amylin can bind to the CTR without any modification of the receptor <ref name="Hay">PMID:26071095</ref>. The two ligands have many conserved residues (highlighted in blue), and this shows that the two ligands share many chemical properties which explains why they are structurally similar. In addition to having many conserved residues, both ligands also have an amidated C-terminus (Figure 3). When the CTR is bound to a RAMP, the complex becomes the AMYR and has greater affinity for the <scene name='10/1037495/Ligand_in_membrane/2'>amylin ligand</scene> relative to the calcitonin ligand. Therefore, the RAMP is essential to AMYR because it causes AMYR to have greater affinity for the amylin ligand rather than the calcitonin ligand. [[Image:Sequence alignment ligands.png|500px|left|thumb|'''Figure 3.''' Sequence alignment of rat amylin and salmon calcitonin. Conserved residues are highlighted in blue.]] |
== Ligands == | == Ligands == | ||
| Line 39: | Line 39: | ||
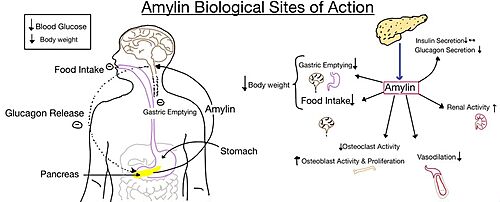
[[Image:Amylin biological role.jpg|500 px|right|thumb|'''Figure 4.''' Different effects of amylin in the human body.]] | [[Image:Amylin biological role.jpg|500 px|right|thumb|'''Figure 4.''' Different effects of amylin in the human body.]] | ||
| - | The functional pharmacology of AMYRs has relied on interference from differences between the behavior of CTRs in the presence and absence of RAMPs. Thus, understanding the structural basis for binding and selectivity of peptides to CTR and AMYRs is important for future drug discovery and development. | + | The functional pharmacology of AMYRs has relied on interference from differences between the behavior of CTRs in the presence and absence of RAMPs <ref name="Hay">PMID:26071095</ref>. Thus, understanding the structural basis for binding and selectivity of peptides to CTR and AMYRs is important for future drug discovery and development. |
===Diabetes=== | ===Diabetes=== | ||
| Line 47: | Line 47: | ||
==== Pramlintide ==== | ==== Pramlintide ==== | ||
[[Image:Pram sequ.png|500px|left|thumb|'''Figure 5.''' Sequence alignment of rat amylin and pramlintide.]] | [[Image:Pram sequ.png|500px|left|thumb|'''Figure 5.''' Sequence alignment of rat amylin and pramlintide.]] | ||
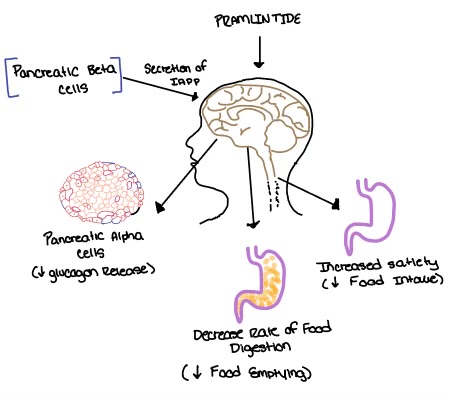
| - | Pramlintide, a peptide analog of human amylin, is FDA-approved for the treatment of insulin-requiring diabetes (Figure 5). Pramlintide is injected into the bloodstream by the beta cells of the pancreas along with insulin after a meal, aiding in the regulation of blood glucose by slowing [https://en.wikipedia.org/wiki/Stomach gastric emptying], promoting [https://en.wikipedia.org/wiki/Satiety satiety] via [https://en.wikipedia.org/wiki/Hypothalamus hypothalamic] receptors, and inhibiting secretion of glucagon which opposes the effects of insulin and amylin (Figure 6). | + | Pramlintide, a peptide analog of human amylin, is FDA-approved for the treatment of insulin-requiring diabetes (Figure 5) <ref name="Hay">PMID:26071095</ref>. Pramlintide is injected into the bloodstream by the beta cells of the pancreas along with insulin after a meal, aiding in the regulation of blood glucose by slowing [https://en.wikipedia.org/wiki/Stomach gastric emptying], promoting [https://en.wikipedia.org/wiki/Satiety satiety] via [https://en.wikipedia.org/wiki/Hypothalamus hypothalamic] receptors, and inhibiting secretion of glucagon which opposes the effects of insulin and amylin (Figure 6) <ref name="Thapa">Thapa, G., Kumari, A., Dasgupta, D., Bandyopadhy, S., Sarkar, N., Roy, K., Karunakaran, G., Kazmi, I., Karmakar, S., & Chakraborty, M. (2023). Chapter 5- Insight into the mechanism of action of anti-diabetic drugs. ''How Synthetic Drugs Work.'' 95-122. [http://dx.doi.org/10.1016/B978-0-323-99855-0.00005-1 DOI:10.1016/B978-0-323-99855-0.00005-1]</ref>. |
[[Image:Pramlintide.jpeg|500 px|right|thumb|'''Figure 6.''' Pramlintide's effect on the human body. Pramlintide is a peptide agonist of human amylin and is a FDA approved treatment for diabetes.]] | [[Image:Pramlintide.jpeg|500 px|right|thumb|'''Figure 6.''' Pramlintide's effect on the human body. Pramlintide is a peptide agonist of human amylin and is a FDA approved treatment for diabetes.]] | ||
| Line 57: | Line 57: | ||
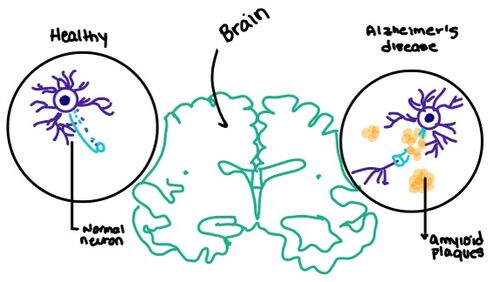
| - | [https://en.wikipedia.org/wiki/Alzheimer%27s_disease Alzheimer's] is a neurodegenerative disease that is commonly associated with the slow progression of amyloid plaque build-up within the gray matter of the aging brain. [https://en.wikipedia.org/wiki/Amyloid_plaques Amyloid Plaques], also known as neuritic plaques or senile plaques, are extracellular deposits of the [https://en.wikipedia.org/wiki/Amyloid_beta amyloid beta protein] that vary in both size and shape with the ability to clump together. When abnormal levels of amylin containing plaques clump together, it creates deposits within the brain region to disrupt proper cell function (Figure 7.). Understanding this disruption is important due the structural overlap seen with amylin and calcitonin binding sites. It can be hypothesized that the conformational similarities between the receptor bringing regions is a key proponent in amyloid plaque build-up and neurodegenerative issues. | + | [https://en.wikipedia.org/wiki/Alzheimer%27s_disease Alzheimer's] is a neurodegenerative disease that is commonly associated with the slow progression of amyloid plaque build-up within the gray matter of the aging brain. [https://en.wikipedia.org/wiki/Amyloid_plaques Amyloid Plaques], also known as neuritic plaques or senile plaques, are extracellular deposits of the [https://en.wikipedia.org/wiki/Amyloid_beta amyloid beta protein] that vary in both size and shape with the ability to clump together <ref name="Press">Press, M., Jung, T., Konig, J., Grune, T., & Hohn, A. (2019). Protein aggregates and proteostasis in aging: Amylin and β-cell function. ''Mechanisms of Ageing and Development. 3,'' 46-54. [http://dx.doi.org/10.1016/j.mad.2018.03.010 DOI:10.1016/j.mad.2018.03.010]</ref>. When abnormal levels of amylin containing plaques clump together, it creates deposits within the brain region to disrupt proper cell function (Figure 7.). Understanding this disruption is important due the structural overlap seen with amylin and calcitonin binding sites. It can be hypothesized that the conformational similarities between the receptor bringing regions is a key proponent in amyloid plaque build-up and neurodegenerative issues. |
| - | |||
| - | |||
| - | <ref name="Ransey">PMID:28504306</ref> | ||
| Line 68: | Line 65: | ||
<references/> | <references/> | ||
| - | <ref name="Ransey"/> | ||
<ref name="7TYF"/> | <ref name="7TYF"/> | ||
| + | <ref name="Hay"> | ||
| + | <ref name="Thapa"> | ||
| + | <ref name="Press"> | ||
== Student Contributors == | == Student Contributors == | ||
Revision as of 21:58, 24 April 2024
Amylin Receptor (AMYR)
| |||||||||||
References
- ↑ Cao J, Belousoff MJ, Liang YL, Johnson RM, Josephs TM, Fletcher MM, Christopoulos A, Hay DL, Danev R, Wootten D, Sexton PM. A structural basis for amylin receptor phenotype. Science. 2022 Mar 25;375(6587):eabm9609. PMID:35324283 doi:10.1126/science.abm9609
- ↑ 2.0 2.1 2.2 2.3 2.4 Hay DL, Chen S, Lutz TA, Parkes DG, Roth JD. Amylin: Pharmacology, Physiology, and Clinical Potential. Pharmacol Rev. 2015 Jul;67(3):564-600. PMID:26071095 doi:10.1124/pr.115.010629
- ↑ Thapa, G., Kumari, A., Dasgupta, D., Bandyopadhy, S., Sarkar, N., Roy, K., Karunakaran, G., Kazmi, I., Karmakar, S., & Chakraborty, M. (2023). Chapter 5- Insight into the mechanism of action of anti-diabetic drugs. How Synthetic Drugs Work. 95-122. DOI:10.1016/B978-0-323-99855-0.00005-1
- ↑ Press, M., Jung, T., Konig, J., Grune, T., & Hohn, A. (2019). Protein aggregates and proteostasis in aging: Amylin and β-cell function. Mechanisms of Ageing and Development. 3, 46-54. DOI:10.1016/j.mad.2018.03.010