User:Jaelin Lunato/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 19: | Line 19: | ||
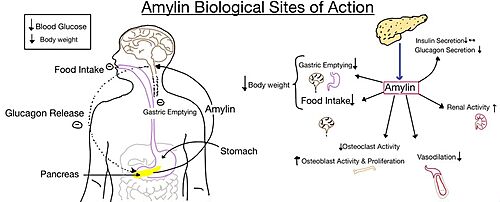
The two major ligands of the calcitonin receptor are [https://en.wikipedia.org/wiki/Calcitonin calcitonin] and [https://en.wikipedia.org/wiki/Amylin amylin]. Calcitonin is a peptide hormone secreted from the thyroid, and it is involved in the regulation of calcium and phosphate in the blood. Amylin is also a hormone and is secreted by pancreatic beta cells. Amylin binding activates many different biological processes affecting different systems with the human body. For example, amylin binding can affect the immune system, the central nervous system, and the satiation system in the brain (i.e., the area postrema).<ref name="Press"> Press, M., Jung, T., Konig, J., Grune, T., & Hohn, A. (2019) Protein aggregates and proteostasis in aging: Amylin and β-cell function. ''Mechanisms of Ageing and Development. 3,'' 46-54. [http://dx.doi.org/10.1016/j.mad.2018.03.010 DOI:10.1016/j.mad.2018.03.010]</ref> | The two major ligands of the calcitonin receptor are [https://en.wikipedia.org/wiki/Calcitonin calcitonin] and [https://en.wikipedia.org/wiki/Amylin amylin]. Calcitonin is a peptide hormone secreted from the thyroid, and it is involved in the regulation of calcium and phosphate in the blood. Amylin is also a hormone and is secreted by pancreatic beta cells. Amylin binding activates many different biological processes affecting different systems with the human body. For example, amylin binding can affect the immune system, the central nervous system, and the satiation system in the brain (i.e., the area postrema).<ref name="Press"> Press, M., Jung, T., Konig, J., Grune, T., & Hohn, A. (2019) Protein aggregates and proteostasis in aging: Amylin and β-cell function. ''Mechanisms of Ageing and Development. 3,'' 46-54. [http://dx.doi.org/10.1016/j.mad.2018.03.010 DOI:10.1016/j.mad.2018.03.010]</ref> | ||
| - | There are two required post-translational modifications of amylin in order for the ligand to have any bioactivity: (1) <scene name='10/1037495/C-term_amide/2'>amidation of the C-terminus</scene> and (2) a <scene name='10/1037495/Amylin_disulfide_bond2/4'>disulfide bond</scene> between C2 and C7. The amidated C-terminus of amylin has a nitrogen present which makes three significant hydrogen bonds. There is one hydrogen bond between the end of amylin and the backbone of the CTR and two hydrogen bonds back to the backbone of amylin itself. The amidated C-terminus is essential to the bioactivity of amylin because the | + | There are two required post-translational modifications of amylin in order for the ligand to have any bioactivity: (1) <scene name='10/1037495/C-term_amide/2'>amidation of the C-terminus</scene> and (2) a <scene name='10/1037495/Amylin_disulfide_bond2/4'>disulfide bond</scene> between C2 and C7. The amidated C-terminus of amylin has a nitrogen present which makes three significant hydrogen bonds. There is one hydrogen bond between the end of amylin and the backbone of the CTR and two hydrogen bonds back to the backbone of amylin itself. The amidated C-terminus is essential to the bioactivity of amylin because the nitrogen participates in Hydrogen bonding and holds the amylin ligand in a specific orientation. Additionally, the hydrogen bonds orient the amylin Y37 residue in a specific orientation so the Y37 side chain can participate in non-polar interactions in the space opposite of the hydrogen bonds. The disulfide bonds between C2 and C7 inhibit the end of amylin from waving around freely while conserving the N-terminus loop structure. Additionally, it prevents amylin from aggregating which would affect amylin binding to the receptor.<ref name="Cao">PMID:35324283</ref> |
== 12 Angstrom Calcitonin Shift == | == 12 Angstrom Calcitonin Shift == | ||
| Line 29: | Line 29: | ||
== Binding Site Interactions == | == Binding Site Interactions == | ||
| - | There are <scene name='10/1037496/Amylin_2hbonds/4'>two conserved hydrogen bonds between the CTR and the amylin N-terminus loop</scene>. These bonds contribute to the functional phenotype of AMYR and also causes the end of the amylin ligand to be held in a flipped up position. | + | There are <scene name='10/1037496/Amylin_2hbonds/4'>two conserved hydrogen bonds between the CTR and the amylin N-terminus loop</scene>. These bonds contribute to the functional phenotype of AMYR and also causes the end of the amylin ligand to be held in a "flipped up", looped position. The hydrogen bond between amylin T6 and the CTR H302 is a conserved interaction across all peptides in the calcitonin family, and it is involved in later production of cAMP. |
| + | Additionally, substituting T6 to A causes a large reduction in response to signaling. <ref name="Cao">PMID:35324283</ref> | ||
There are water molecules present in the binding site between amylin and the calcitonin receptor that support the ligand-receptor interaction. Some water molecules interact with the amylin ligand and create water-bridged Hydrogen bonds between different ligand residues, such as the <scene name='10/1037495/Water_1_ver3/4'>water-bridged Hydrogen bond between the main chains of T6 and T9</scene>. Other water molecules create <scene name='10/1037496/Water_receptor/1'>water-bridged Hydrogen bonds between residues of the calcitonin receptor</scene>. The water molecules are present in the empty space located in the ligand binding site, and they are hypothesized to stabilize the active conformation of the calcitonin receptor when amylin is bound. Substitutions of polar residues involved with the water-bridged Hydrogen bond network to nonpolar residues causes a decrease in potency and affinity of amylin to the calcitonin receptor.<ref name="Cao">PMID:35324283</ref> | There are water molecules present in the binding site between amylin and the calcitonin receptor that support the ligand-receptor interaction. Some water molecules interact with the amylin ligand and create water-bridged Hydrogen bonds between different ligand residues, such as the <scene name='10/1037495/Water_1_ver3/4'>water-bridged Hydrogen bond between the main chains of T6 and T9</scene>. Other water molecules create <scene name='10/1037496/Water_receptor/1'>water-bridged Hydrogen bonds between residues of the calcitonin receptor</scene>. The water molecules are present in the empty space located in the ligand binding site, and they are hypothesized to stabilize the active conformation of the calcitonin receptor when amylin is bound. Substitutions of polar residues involved with the water-bridged Hydrogen bond network to nonpolar residues causes a decrease in potency and affinity of amylin to the calcitonin receptor.<ref name="Cao">PMID:35324283</ref> | ||
== G Protein Activation == | == G Protein Activation == | ||
| - | There are extensive nonpolar, <scene name='10/1037496/H2ophobic_interactions/2'>hydrophobic interactions between the G protein Gα subunit and the CTR 2nd intracellular loop</scene>. | + | There are extensive nonpolar, <scene name='10/1037496/H2ophobic_interactions/2'>hydrophobic interactions between the G protein Gα subunit and the CTR 2nd intracellular loop</scene>. V322 and L323 of the CTR 2nd intracellular loop makes nonpolar interactions with I248 and V249 back to itself as well as L388 of the Gα subunit. These interactions stabilize the calcitonin receptor, which activates the G-protein to give amylin its biological role.<ref name="Cao">PMID:35324283</ref> The <scene name='10/1037495/G_protein_interaction/4'>CTR 3rd intracellular loop interacts with the Gα subunit through Hydrogen bonding</scene>. R180 of the 3rd intracellular loop of the calcitonin receptor makes a crucial hydrogen bond to Q384 of the Gα subunit. This interaction has been shown to maintain the biological function of amylin through cell signaling.<ref name="Cao">PMID:35324283</ref> These interactions between the second and third intracellular loops of the calcitonin receptor stabilize the conformational states of the G-protein to create a more rigid domain with the nanobody. |
== Biological Relevance == | == Biological Relevance == | ||
| Line 42: | Line 43: | ||
===Diabetes=== | ===Diabetes=== | ||
| - | |||
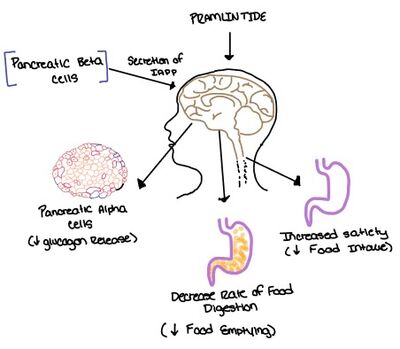
Amylin, as it is a part of the calcitonin peptide family, is heavily related to the regulation of homeostatic processes to relevant drug targets. Amylin is the target for the treatment of [https://en.wikipedia.org/wiki/Diabetes diabetes]. Amylin is a neuroendocrine hormone that is synthesized and co-secreted with insulin. [https://en.wikipedia.org/wiki/Insulin Insulin] triggers glucose uptake which removes glucose from the bloodstream using it then for energy. Amylin works in negatively regulating (inhibiting) the formation of [https://en.wikipedia.org/wiki/Glucagon glucagon] so that glucose polymers can continue to be broken down into the bloodstream for further energy storage and consumption. Therefore with the co-secretion of both amylin and insulin, it would aid in decreasing blood glucose levels thus becoming a predominant treatment plan for diabetic disorders, such as shown with the developing drug [https://en.wikipedia.org/wiki/Pramlintide Pramlintide].<ref name="Mathiesen">PMID:33488526</ref> | Amylin, as it is a part of the calcitonin peptide family, is heavily related to the regulation of homeostatic processes to relevant drug targets. Amylin is the target for the treatment of [https://en.wikipedia.org/wiki/Diabetes diabetes]. Amylin is a neuroendocrine hormone that is synthesized and co-secreted with insulin. [https://en.wikipedia.org/wiki/Insulin Insulin] triggers glucose uptake which removes glucose from the bloodstream using it then for energy. Amylin works in negatively regulating (inhibiting) the formation of [https://en.wikipedia.org/wiki/Glucagon glucagon] so that glucose polymers can continue to be broken down into the bloodstream for further energy storage and consumption. Therefore with the co-secretion of both amylin and insulin, it would aid in decreasing blood glucose levels thus becoming a predominant treatment plan for diabetic disorders, such as shown with the developing drug [https://en.wikipedia.org/wiki/Pramlintide Pramlintide].<ref name="Mathiesen">PMID:33488526</ref> | ||
| Line 55: | Line 55: | ||
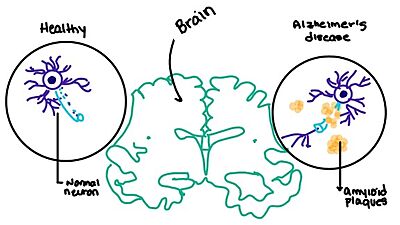
[[Image:Amylin brain.jpeg|400 px|right|thumb|'''Figure 7.''' Amylin's effect on the brain through the buildup of amyloid plaques.]] | [[Image:Amylin brain.jpeg|400 px|right|thumb|'''Figure 7.''' Amylin's effect on the brain through the buildup of amyloid plaques.]] | ||
| - | |||
| - | |||
[https://en.wikipedia.org/wiki/Alzheimer%27s_disease Alzheimer's] is a neurodegenerative disease that is commonly associated with the slow progression of amyloid plaque build-up within the gray matter of the aging brain. [https://en.wikipedia.org/wiki/Amyloid_plaques Amyloid Plaques], also known as neuritic plaques or senile plaques, are extracellular deposits of the [https://en.wikipedia.org/wiki/Amyloid_beta amyloid beta protein] that vary in both size and shape with the ability to clump together.<ref name="Press">Press, M., Jung, T., Konig, J., Grune, T., & Hohn, A. (2019). Protein aggregates and proteostasis in aging: Amylin and β-cell function. ''Mechanisms of Ageing and Development. 3,'' 46-54. [http://dx.doi.org/10.1016/j.mad.2018.03.010 DOI:10.1016/j.mad.2018.03.010]</ref> When abnormal levels of amylin containing plaques clump together, it creates deposits within the brain region to disrupt proper cell function (Figure 7).<ref name="Grizzanti">PMID:30282360</ref> Understanding this disruption is important due the structural overlap seen with amylin and calcitonin binding sites. It can be hypothesized that the conformational similarities between the receptor bringing regions is a key proponent in amyloid plaque build-up and neurodegenerative issues. | [https://en.wikipedia.org/wiki/Alzheimer%27s_disease Alzheimer's] is a neurodegenerative disease that is commonly associated with the slow progression of amyloid plaque build-up within the gray matter of the aging brain. [https://en.wikipedia.org/wiki/Amyloid_plaques Amyloid Plaques], also known as neuritic plaques or senile plaques, are extracellular deposits of the [https://en.wikipedia.org/wiki/Amyloid_beta amyloid beta protein] that vary in both size and shape with the ability to clump together.<ref name="Press">Press, M., Jung, T., Konig, J., Grune, T., & Hohn, A. (2019). Protein aggregates and proteostasis in aging: Amylin and β-cell function. ''Mechanisms of Ageing and Development. 3,'' 46-54. [http://dx.doi.org/10.1016/j.mad.2018.03.010 DOI:10.1016/j.mad.2018.03.010]</ref> When abnormal levels of amylin containing plaques clump together, it creates deposits within the brain region to disrupt proper cell function (Figure 7).<ref name="Grizzanti">PMID:30282360</ref> Understanding this disruption is important due the structural overlap seen with amylin and calcitonin binding sites. It can be hypothesized that the conformational similarities between the receptor bringing regions is a key proponent in amyloid plaque build-up and neurodegenerative issues. | ||
| - | |||
| - | |||
</StructureSection> | </StructureSection> | ||
Revision as of 01:04, 25 April 2024
Amylin Receptor (AMYR)
| |||||||||||
References
- ↑ Cao J, Belousoff MJ, Liang YL, Johnson RM, Josephs TM, Fletcher MM, Christopoulos A, Hay DL, Danev R, Wootten D, Sexton PM. A structural basis for amylin receptor phenotype. Science. 2022 Mar 25;375(6587):eabm9609. PMID:35324283 doi:10.1126/science.abm9609
- ↑ 2.0 2.1 2.2 2.3 2.4 Hay DL, Chen S, Lutz TA, Parkes DG, Roth JD. Amylin: Pharmacology, Physiology, and Clinical Potential. Pharmacol Rev. 2015 Jul;67(3):564-600. PMID:26071095 doi:10.1124/pr.115.010629
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 Cao J, Belousoff MJ, Liang YL, Johnson RM, Josephs TM, Fletcher MM, Christopoulos A, Hay DL, Danev R, Wootten D, Sexton PM. A structural basis for amylin receptor phenotype. Science. 2022 Mar 25;375(6587):eabm9609. PMID:35324283 doi:10.1126/science.abm9609
- ↑ 4.0 4.1 Press, M., Jung, T., Konig, J., Grune, T., & Hohn, A. (2019) Protein aggregates and proteostasis in aging: Amylin and β-cell function. Mechanisms of Ageing and Development. 3, 46-54. DOI:10.1016/j.mad.2018.03.010
- ↑ Mathiesen DS, Lund A, Vilsbøll T, Knop FK, Bagger JI. Amylin and Calcitonin: Potential Therapeutic Strategies to Reduce Body Weight and Liver Fat. Front Endocrinol (Lausanne). 2021 Jan 8;11:617400. PMID:33488526 doi:10.3389/fendo.2020.617400
- ↑ Thapa, G., Kumari, A., Dasgupta, D., Bandyopadhy, S., Sarkar, N., Roy, K., Karunakaran, G., Kazmi, I., Karmakar, S., & Chakraborty, M. (2023). Chapter 5- Insight into the mechanism of action of anti-diabetic drugs. How Synthetic Drugs Work. 95-122. DOI:10.1016/B978-0-323-99855-0.00005-1
- ↑ Grizzanti J, Corrigan R, Casadesus G. Neuroprotective Effects of Amylin Analogues on Alzheimer's Disease Pathogenesis and Cognition. J Alzheimers Dis. 2018;66(1):11-23. PMID:30282360 doi:10.3233/JAD-180433