We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Chloe Tucker/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 19: | Line 19: | ||
[[Image:GIP_hydrogen_bonds.jpg|350 px|right|thumb|Figure 1. GIPR and GIP residue interactions]] | [[Image:GIP_hydrogen_bonds.jpg|350 px|right|thumb|Figure 1. GIPR and GIP residue interactions]] | ||
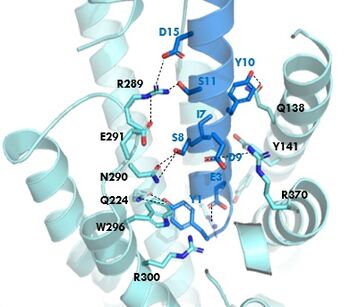
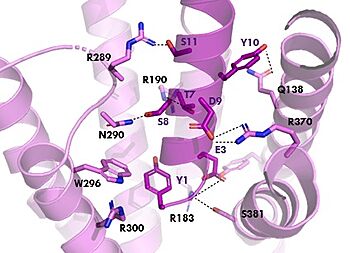
The <scene name='10/1038815/Overview/3'>binding site</scene> of GIP with the GIP receptor (GIPR) is where the N-term of GIP binds with the transmembrane domain of the GIPR. The first interaction formed with GIPR and the N-term of GIP is a hydrogen bond between Tyrosine 1 (Y1) and Glutamine 224 (Q224) to activate the G-protein to start sending signals to the cell. | The <scene name='10/1038815/Overview/3'>binding site</scene> of GIP with the GIP receptor (GIPR) is where the N-term of GIP binds with the transmembrane domain of the GIPR. The first interaction formed with GIPR and the N-term of GIP is a hydrogen bond between Tyrosine 1 (Y1) and Glutamine 224 (Q224) to activate the G-protein to start sending signals to the cell. | ||
| - | Many other <scene name='10/1038815/Active_site/3'>residues</scene> within the binding site are forming hydrogen bonds and hydrophobic interactions between the ligand and the receptor. The N-term binds more strongly than the C-term and there are many different residues contributing to this, including, Tyrosine 1 (Y1) and Tryptophan (W296) are forming aromatic interactions. Serine 8 (S8) and Asparagine 290 (N290) are forming two hydrogen bonds with each other. Aspartate 9 (D9) is forming another hydrogen bond with Arginine 370 (R370)<ref name= | + | Many other <scene name='10/1038815/Active_site/3'>residues</scene> within the binding site are forming hydrogen bonds and hydrophobic interactions between the ligand and the receptor. The N-term binds more strongly than the C-term and there are many different residues contributing to this, including, Tyrosine 1 (Y1) and Tryptophan (W296) are forming aromatic interactions. Serine 8 (S8) and Asparagine 290 (N290) are forming two hydrogen bonds with each other. Aspartate 9 (D9) is forming another hydrogen bond with Arginine 370 (R370)<ref name="Sun"/>. These hydrogen bonds lead to the activation of cell signaling and when this binding is somehow disrupted, that is what causes different diseases like diabetes. |
=== Binding/Active Site of GIPR with Tirzepatide === | === Binding/Active Site of GIPR with Tirzepatide === | ||
| Line 31: | Line 31: | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
| - | < | + | <ref name="Sun">PMID:35333651</ref> |
== Student Contributors == | == Student Contributors == | ||
*Chloe Tucker | *Chloe Tucker | ||
*Mandy Bechman | *Mandy Bechman | ||
Revision as of 13:51, 25 April 2024
GIP and GIP-R
| |||||||||||
References
Student Contributors
- Chloe Tucker
- Mandy Bechman