We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Chloe Tucker/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 24: | Line 24: | ||
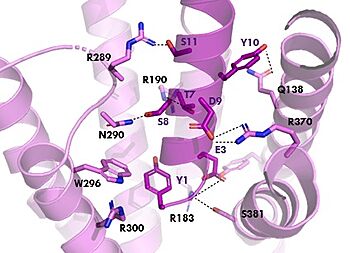
[[Image:TZ_hydrogen_bonds.jpg|350 px|right|thumb|Figure 2. Residue Interactions with Tirzepatide]] | [[Image:TZ_hydrogen_bonds.jpg|350 px|right|thumb|Figure 2. Residue Interactions with Tirzepatide]] | ||
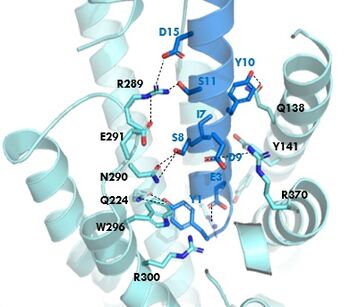
The <scene name='10/1038815/Tirzepatide_active_site/2'>Tirzepatide binding site</scene> is the same as GIP with the N-term binding to the transmembrane domain. | The <scene name='10/1038815/Tirzepatide_active_site/2'>Tirzepatide binding site</scene> is the same as GIP with the N-term binding to the transmembrane domain. | ||
| - | The <scene name='10/1038815/Tirzepatide_residues/5'>residues</scene> are fairly similar just in a different conformations, which is allowing for more hydrogen bonding. The more hydrogen bonding there is the stronger the binding between the two proteins will be. In Tirzepatide the most noticeable change is the Tyrosine 1 (Y1) residue. It is now facing up towards Arginine 190 (R190) where they | + | The <scene name='10/1038815/Tirzepatide_residues/5'>residues</scene> are fairly similar just in a different conformations, which is allowing for more hydrogen bonding. The more hydrogen bonding there is the stronger the binding between the two proteins will be. In Tirzepatide, the most noticeable conformational change is the Tyrosine 1 (Y1) residue. It is now facing up towards Arginine 190 (R190) where they are now form a new hydrogen bond that was not present in GIP. This can lead to the conclusion that GIPR has a higher binding affinity for Tirzepatide than GIP itself<ref name="Sun"/>. |
=== Isoleucine vs. Threonine === | === Isoleucine vs. Threonine === | ||
<scene name='10/1038815/Tirzepatide_thr7/2'>Threonine 7</scene> | <scene name='10/1038815/Tirzepatide_thr7/2'>Threonine 7</scene> | ||
Revision as of 14:01, 25 April 2024
GIP and GIP-R
| |||||||||||
References
Student Contributors
- Chloe Tucker
- Mandy Bechman