We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Ben Whiteside
From Proteopedia
(Difference between revisions)
| Line 11: | Line 11: | ||
====Amidated C-Terminus==== | ====Amidated C-Terminus==== | ||
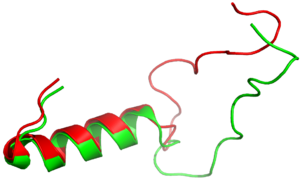
The <scene name='10/1038819/Amidated_c_term/9'>C-Terminus</scene> of amylin contains an amide group, rather than a carboxylic acid group. This chemical modification allows for more extensive hydrogen bonding to nearby residues, due to the added hydrogen bond donor on the NH2 group. In turn, this allows for favorable hydrogen bonds between S129 of the transmembrane domain and the main chain of Y37 on amylin. This interaction causes a "kink" in the random coil of amylin, displacing Y37 into a hydrophobic pocket, allowing for favorable hydrophobic interactions with W79 of the transmembrane domain. This amidation is thought to be a post-translational modification. | The <scene name='10/1038819/Amidated_c_term/9'>C-Terminus</scene> of amylin contains an amide group, rather than a carboxylic acid group. This chemical modification allows for more extensive hydrogen bonding to nearby residues, due to the added hydrogen bond donor on the NH2 group. In turn, this allows for favorable hydrogen bonds between S129 of the transmembrane domain and the main chain of Y37 on amylin. This interaction causes a "kink" in the random coil of amylin, displacing Y37 into a hydrophobic pocket, allowing for favorable hydrophobic interactions with W79 of the transmembrane domain. This amidation is thought to be a post-translational modification. | ||
| + | === Bypass Motif === | ||
| + | === G-alpha Interactions with CTR TMD === | ||
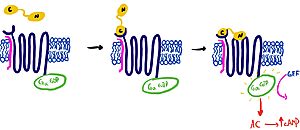
| + | To transduce the signal across the cell membrane, the binding of amylin will induce a conformational change that allows for the CTR to make favorable interactions with the G alpha subunit. Two interactions shown <scene name='10/1038828/Ctr_g_alpha/15'>(1, </scene><scene name='10/1038828/Ctr_g_alpha/12'>2) </scene> activate the G-protein and propel downstream signaling. As with a typical glucagon GPCR pathway, the activated G-alpha subunit becomes activated upon guanine exchange factor [https://en.wikipedia.org/wiki/Guanine_nucleotide_exchange_factor (GEF)] activity. This G-alpha subunit transverses laterally in the membrane towards adenylyl cyclase, activating it and causing an increase in the second messenger cyclic adenosine monophosphate [https://en.wikipedia.org/wiki/Cyclic_adenosine_monophosphate (cAMP)]. This cAMP activates protein kinase A [https://en.wikipedia.org/wiki/Protein_kinase_A (PKA)], which can phosphorylate other proteins facilitating cellular response. | ||
| + | |||
==Amylin Receptor Binding== | ==Amylin Receptor Binding== | ||
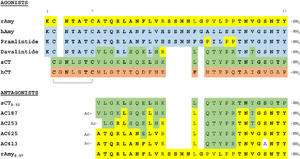
=== Two-Domain Model of Amylin Binding === | === Two-Domain Model of Amylin Binding === | ||
| Line 18: | Line 22: | ||
==== Extracellular Domain - RAMP interactions ==== | ==== Extracellular Domain - RAMP interactions ==== | ||
The extracellular domain of the CTR primarily contains polar residues in the extracellular space. In order to orient these residues in such a way to facilitate amylin binding, RAMP makes hydrogen bonds with the CTR to increase the rigidity of the receptor binding site. <scene name='10/1038828/Ctr_ramp_ecd_stablization/7'>RAMP CTR Extracellular Domain Interaction</scene> | The extracellular domain of the CTR primarily contains polar residues in the extracellular space. In order to orient these residues in such a way to facilitate amylin binding, RAMP makes hydrogen bonds with the CTR to increase the rigidity of the receptor binding site. <scene name='10/1038828/Ctr_ramp_ecd_stablization/7'>RAMP CTR Extracellular Domain Interaction</scene> | ||
| - | === Bypass Motif === | ||
| - | === G-alpha Interactions with CTR TMD === | ||
| - | To transduce the signal across the cell membrane, the binding of amylin will induce a conformational change that allows for the CTR to make favorable interactions with the G alpha subunit. Two interactions shown <scene name='10/1038828/Ctr_g_alpha/15'>(1, </scene><scene name='10/1038828/Ctr_g_alpha/12'>2) </scene> activate the G-protein and propel downstream signaling. As with a typical glucagon GPCR pathway, the activated G-alpha subunit becomes activated upon guanine exchange factor [https://en.wikipedia.org/wiki/Guanine_nucleotide_exchange_factor (GEF)] activity. This G-alpha subunit transverses laterally in the membrane towards adenylyl cyclase, activating it and causing an increase in the second messenger cyclic adenosine monophosphate [https://en.wikipedia.org/wiki/Cyclic_adenosine_monophosphate (cAMP)]. This cAMP activates protein kinase A [https://en.wikipedia.org/wiki/Protein_kinase_A (PKA)], which can phosphorylate other proteins facilitating cellular response. | ||
| - | |||
== Clinical Significance == | == Clinical Significance == | ||
Revision as of 14:31, 25 April 2024
AMYR
| |||||||||||
Student Contributors
Andrew Helmerich, Mathias Vander Eide, Ben Whiteside