User:Jaelin Lunato/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 22: | Line 22: | ||
== Calcitonin 12 Angstrom Shift == | == Calcitonin 12 Angstrom Shift == | ||
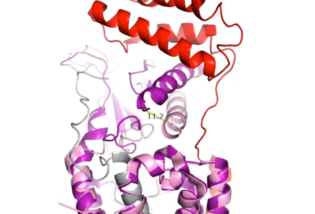
| - | When the RAMP binds to the CTR, the CTR undergoes a conformational change, or shift. The extracellular region of the calcitonin subunit, specifically in the C-alpha loop 5, of AMYR will undergo a 12 angstrom shift upwards.<ref name="Cao">PMID:35324283</ref> This distance shift is measured specifically from a glutamate residue, E123. This shift in the CTR facilitates the packing of the CTR extracellular domain with the antiparallel triple helix conformation of the RAMP extracellular domain. | + | [[Image:12ang.png|315 px|thumb|right| '''Figure 4.''' Active Site 12 Angstrom Shift of Receptor by RAMP Binding]] When the RAMP binds to the CTR, the CTR undergoes a conformational change, or shift. The extracellular region of the calcitonin subunit, specifically in the C-alpha loop 5, of AMYR will undergo a 12 angstrom shift upwards (Figure 4).<ref name="Cao">PMID:35324283</ref> This distance shift is measured specifically from a glutamate residue, E123. This shift in the CTR facilitates the packing of the CTR extracellular domain with the antiparallel triple helix conformation of the RAMP extracellular domain. |
| - | + | ||
| - | + | ||
There is little movement in the N-terminal location and midregion of the CTR when RAMP binds. This is because there is an unstructured and flexible "hinge" region between the extracellular domain and the core-binding domains, allowing these domains to remain in their original location from before RAMP binds to the CTR. | There is little movement in the N-terminal location and midregion of the CTR when RAMP binds. This is because there is an unstructured and flexible "hinge" region between the extracellular domain and the core-binding domains, allowing these domains to remain in their original location from before RAMP binds to the CTR. | ||
| Line 37: | Line 35: | ||
== Biological Relevance == | == Biological Relevance == | ||
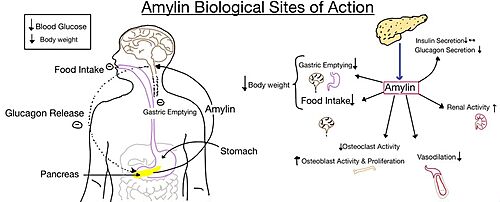
| - | [[Image:Amylin biological role.jpg|500 px|right|thumb|'''Figure | + | [[Image:Amylin biological role.jpg|500 px|right|thumb|'''Figure 5.''' Different effects of amylin in the human body.]] |
The functional pharmacology of AMYRs has relied on interference from differences between the behavior of CTRs in the presence and absence of RAMPs.<ref name="Hay">PMID:26071095</ref> Thus, understanding the structural basis for binding and selectivity of peptides to CTR and AMYRs is important for future drug discovery and development. | The functional pharmacology of AMYRs has relied on interference from differences between the behavior of CTRs in the presence and absence of RAMPs.<ref name="Hay">PMID:26071095</ref> Thus, understanding the structural basis for binding and selectivity of peptides to CTR and AMYRs is important for future drug discovery and development. | ||
| Line 45: | Line 43: | ||
==== Pramlintide ==== | ==== Pramlintide ==== | ||
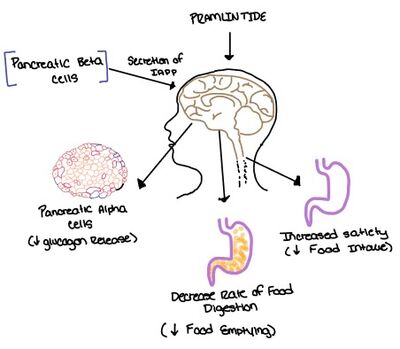
| - | [[Image:Pram sequ.png|500px|right|thumb|'''Figure | + | [[Image:Pram sequ.png|500px|right|thumb|'''Figure 6.''' Sequence alignment of rat amylin and pramlintide.]][[Image:Pramlintide.jpeg|400 px|right|thumb|'''Figure 7.''' Pramlintide's effect on the human body. Pramlintide is a peptide agonist of human amylin and is a FDA approved treatment for diabetes.]] |
| - | Pramlintide, a peptide analog of human amylin, is FDA-approved for the treatment of insulin-requiring diabetes (Figure | + | |
| - | + | Pramlintide, a peptide analog of human amylin, is FDA-approved for the treatment of insulin-requiring diabetes (Figure 6).<ref name="Hay">PMID:26071095</ref> Pramlintide is injected into the bloodstream by the beta cells of the pancreas along with insulin after a meal, aiding in the regulation of blood glucose by slowing [https://en.wikipedia.org/wiki/Stomach gastric emptying], promoting [https://en.wikipedia.org/wiki/Satiety satiety] via [https://en.wikipedia.org/wiki/Hypothalamus hypothalamic] receptors, and inhibiting secretion of glucagon which opposes the effects of insulin and amylin (Figure 7).<ref name="Thapa">Thapa, G., Kumari, A., Dasgupta, D., Bandyopadhy, S., Sarkar, N., Roy, K., Karunakaran, G., Kazmi, I., Karmakar, S., & Chakraborty, M. (2023). Chapter 5- Insight into the mechanism of action of anti-diabetic drugs. ''How Synthetic Drugs Work.'' 95-122. [http://dx.doi.org/10.1016/B978-0-323-99855-0.00005-1 DOI:10.1016/B978-0-323-99855-0.00005-1]</ref> | |
===Alzheimer's=== | ===Alzheimer's=== | ||
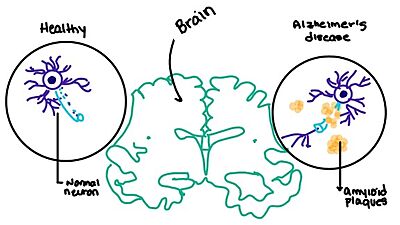
| - | [https://en.wikipedia.org/wiki/Alzheimer%27s_disease Alzheimer's] is a neurodegenerative disease that is commonly associated with the slow progression of amyloid plaque build-up within the gray matter of the aging brain. [https://en.wikipedia.org/wiki/Amyloid_plaques Amyloid plaques], also known as neuritic plaques or senile plaques, are extracellular deposits of the [https://en.wikipedia.org/wiki/Amyloid_beta amyloid beta protein] that vary in both size and shape with the ability to clump together.<ref name="Press">Press, M., Jung, T., Konig, J., Grune, T., & Hohn, A. (2019). Protein aggregates and proteostasis in aging: Amylin and β-cell function. ''Mechanisms of Ageing and Development. 3,'' 46-54. [http://dx.doi.org/10.1016/j.mad.2018.03.010 DOI:10.1016/j.mad.2018.03.010]</ref> When abnormal levels of amylin containing plaques clump together, it creates deposits within the brain region to disrupt proper cell function (Figure | + | [https://en.wikipedia.org/wiki/Alzheimer%27s_disease Alzheimer's] is a neurodegenerative disease that is commonly associated with the slow progression of amyloid plaque build-up within the gray matter of the aging brain. [https://en.wikipedia.org/wiki/Amyloid_plaques Amyloid plaques], also known as neuritic plaques or senile plaques, are extracellular deposits of the [https://en.wikipedia.org/wiki/Amyloid_beta amyloid beta protein] that vary in both size and shape with the ability to clump together.<ref name="Press">Press, M., Jung, T., Konig, J., Grune, T., & Hohn, A. (2019). Protein aggregates and proteostasis in aging: Amylin and β-cell function. ''Mechanisms of Ageing and Development. 3,'' 46-54. [http://dx.doi.org/10.1016/j.mad.2018.03.010 DOI:10.1016/j.mad.2018.03.010]</ref> When abnormal levels of amylin containing plaques clump together, it creates deposits within the brain region to disrupt proper cell function (Figure 8).<ref name="Grizzanti">PMID:30282360</ref> Understanding this disruption is important due the structural overlap seen with amylin and calcitonin binding sites. It can be hypothesized that the conformational similarities between the receptor bringing regions is a key proponent in amyloid plaque build-up and neurodegenerative issues. [[Image:Amylin brain.jpeg|400 px|left|thumb|'''Figure 8.''' Amylin's effect on the brain through the buildup of amyloid plaques.]] |
</StructureSection> | </StructureSection> | ||
Revision as of 00:53, 26 April 2024
Amylin Receptor (AMYR)
| |||||||||||
References
- ↑ Cao J, Belousoff MJ, Liang YL, Johnson RM, Josephs TM, Fletcher MM, Christopoulos A, Hay DL, Danev R, Wootten D, Sexton PM. A structural basis for amylin receptor phenotype. Science. 2022 Mar 25;375(6587):eabm9609. PMID:35324283 doi:10.1126/science.abm9609
- ↑ 2.0 2.1 2.2 2.3 2.4 Hay DL, Chen S, Lutz TA, Parkes DG, Roth JD. Amylin: Pharmacology, Physiology, and Clinical Potential. Pharmacol Rev. 2015 Jul;67(3):564-600. PMID:26071095 doi:10.1124/pr.115.010629
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 Cao J, Belousoff MJ, Liang YL, Johnson RM, Josephs TM, Fletcher MM, Christopoulos A, Hay DL, Danev R, Wootten D, Sexton PM. A structural basis for amylin receptor phenotype. Science. 2022 Mar 25;375(6587):eabm9609. PMID:35324283 doi:10.1126/science.abm9609
- ↑ 4.0 4.1 Press, M., Jung, T., Konig, J., Grune, T., & Hohn, A. (2019) Protein aggregates and proteostasis in aging: Amylin and β-cell function. Mechanisms of Ageing and Development. 3, 46-54. DOI:10.1016/j.mad.2018.03.010
- ↑ Mathiesen DS, Lund A, Vilsbøll T, Knop FK, Bagger JI. Amylin and Calcitonin: Potential Therapeutic Strategies to Reduce Body Weight and Liver Fat. Front Endocrinol (Lausanne). 2021 Jan 8;11:617400. PMID:33488526 doi:10.3389/fendo.2020.617400
- ↑ Thapa, G., Kumari, A., Dasgupta, D., Bandyopadhy, S., Sarkar, N., Roy, K., Karunakaran, G., Kazmi, I., Karmakar, S., & Chakraborty, M. (2023). Chapter 5- Insight into the mechanism of action of anti-diabetic drugs. How Synthetic Drugs Work. 95-122. DOI:10.1016/B978-0-323-99855-0.00005-1
- ↑ Grizzanti J, Corrigan R, Casadesus G. Neuroprotective Effects of Amylin Analogues on Alzheimer's Disease Pathogenesis and Cognition. J Alzheimers Dis. 2018;66(1):11-23. PMID:30282360 doi:10.3233/JAD-180433