User:Jaelin Lunato/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 43: | Line 43: | ||
==== Pramlintide ==== | ==== Pramlintide ==== | ||
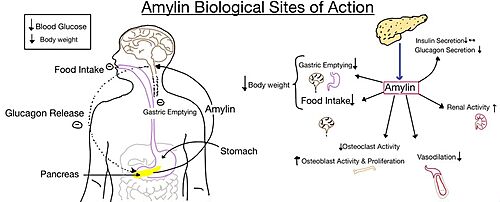
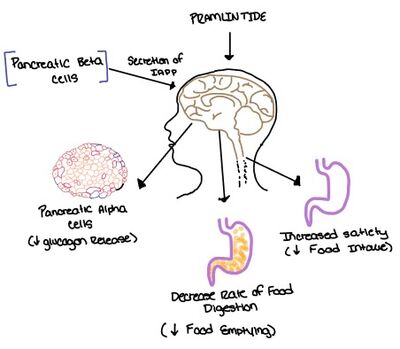
| - | [[Image:Pram sequ.png|500px|right|thumb|'''Figure 6.''' Sequence alignment of rat amylin and pramlintide.]][[Image:Pramlintide.jpeg|400 px|right|thumb|'''Figure 7.''' Pramlintide's effect on the human body. Pramlintide is a peptide agonist of human amylin and is a FDA approved treatment for diabetes.]] | + | [[Image:Pram sequ.png|500px|right|thumb|'''Figure 6.''' Sequence alignment of rat amylin and pramlintide.]][[Image:Pramlintide.jpeg|400 px|right|thumb|'''Figure 7.''' Pramlintide's effect on the human body. Pramlintide is a peptide agonist of human amylin and is a FDA approved treatment for diabetes.]] Pramlintide, a peptide analog of human amylin, is FDA-approved for the treatment of insulin-requiring diabetes. Pramlintide shares many conserved residues with amylin, so the two are chemically and structurally similar (Figure 6).<ref name="Hay">PMID:26071095</ref> Pramlintide is injected into the bloodstream by the beta cells of the pancreas along with insulin after a meal, aiding in the regulation of blood glucose by slowing [https://en.wikipedia.org/wiki/Stomach gastric emptying], promoting [https://en.wikipedia.org/wiki/Satiety satiety] via [https://en.wikipedia.org/wiki/Hypothalamus hypothalamic] receptors, and inhibiting secretion of glucagon which opposes the effects of insulin and amylin (Figure 7).<ref name="Thapa">Thapa, G., Kumari, A., Dasgupta, D., Bandyopadhy, S., Sarkar, N., Roy, K., Karunakaran, G., Kazmi, I., Karmakar, S., & Chakraborty, M. (2023). Chapter 5- Insight into the mechanism of action of anti-diabetic drugs. ''How Synthetic Drugs Work.'' 95-122. [http://dx.doi.org/10.1016/B978-0-323-99855-0.00005-1 DOI:10.1016/B978-0-323-99855-0.00005-1]</ref> |
| - | + | ||
| - | Pramlintide, a peptide analog of human amylin, is FDA-approved for the treatment of insulin-requiring diabetes. Pramlintide shares many conserved residues with amylin, so the two are chemically and structurally similar (Figure 6).<ref name="Hay">PMID:26071095</ref> Pramlintide is injected into the bloodstream by the beta cells of the pancreas along with insulin after a meal, aiding in the regulation of blood glucose by slowing [https://en.wikipedia.org/wiki/Stomach gastric emptying], promoting [https://en.wikipedia.org/wiki/Satiety satiety] via [https://en.wikipedia.org/wiki/Hypothalamus hypothalamic] receptors, and inhibiting secretion of glucagon which opposes the effects of insulin and amylin (Figure 7).<ref name="Thapa">Thapa, G., Kumari, A., Dasgupta, D., Bandyopadhy, S., Sarkar, N., Roy, K., Karunakaran, G., Kazmi, I., Karmakar, S., & Chakraborty, M. (2023). Chapter 5- Insight into the mechanism of action of anti-diabetic drugs. ''How Synthetic Drugs Work.'' 95-122. [http://dx.doi.org/10.1016/B978-0-323-99855-0.00005-1 DOI:10.1016/B978-0-323-99855-0.00005-1]</ref> | + | |
===Alzheimer's=== | ===Alzheimer's=== | ||
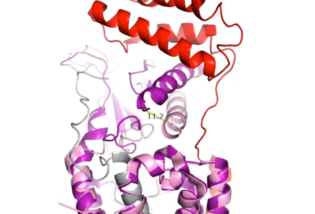
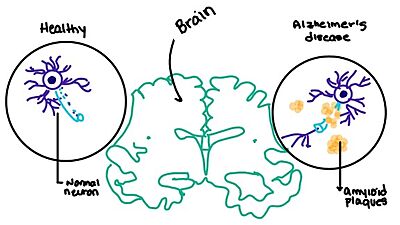
| - | [https://en.wikipedia.org/wiki/Alzheimer%27s_disease Alzheimer's] is a neurodegenerative disease that is commonly associated with the slow progression of amyloid plaque build-up within the gray matter of the aging brain. [https://en.wikipedia.org/wiki/Amyloid_plaques Amyloid plaques], also known as neuritic plaques or senile plaques, are extracellular deposits of the [https://en.wikipedia.org/wiki/Amyloid_beta amyloid beta protein] that vary in both size and shape with the ability to clump together.<ref name="Press">Press, M., Jung, T., Konig, J., Grune, T., & Hohn, A. (2019). Protein aggregates and proteostasis in aging: Amylin and β-cell function. ''Mechanisms of Ageing and Development. 3,'' 46-54. [http://dx.doi.org/10.1016/j.mad.2018.03.010 DOI:10.1016/j.mad.2018.03.010]</ref> When abnormal levels of amylin containing plaques clump together, it creates deposits within the brain region to disrupt proper cell function (Figure 8).<ref name="Grizzanti">PMID:30282360</ref> Understanding this disruption is important due the structural overlap seen with amylin and calcitonin binding sites. It can be hypothesized that the conformational similarities between the receptor | + | [https://en.wikipedia.org/wiki/Alzheimer%27s_disease Alzheimer's] is a neurodegenerative disease that is commonly associated with the slow progression of amyloid plaque build-up within the gray matter of the aging brain. [https://en.wikipedia.org/wiki/Amyloid_plaques Amyloid plaques], also known as neuritic plaques or senile plaques, are extracellular deposits of the [https://en.wikipedia.org/wiki/Amyloid_beta amyloid beta protein] that vary in both size and shape with the ability to clump together.<ref name="Press">Press, M., Jung, T., Konig, J., Grune, T., & Hohn, A. (2019). Protein aggregates and proteostasis in aging: Amylin and β-cell function. ''Mechanisms of Ageing and Development. 3,'' 46-54. [http://dx.doi.org/10.1016/j.mad.2018.03.010 DOI:10.1016/j.mad.2018.03.010]</ref> When abnormal levels of amylin containing plaques clump together, it creates deposits within the brain region to disrupt proper cell function (Figure 8).<ref name="Grizzanti">PMID:30282360</ref> Understanding this disruption is important due to the structural overlap seen with amylin and calcitonin binding sites. It can be hypothesized that the conformational similarities between the receptor binding regions is a key proponent in amyloid plaque build-up and neurodegenerative issues. [[Image:Amylin brain.jpeg|400 px|left|thumb|'''Figure 8.''' Amylin's effect on the brain through the buildup of amyloid plaques.]] |
</StructureSection> | </StructureSection> | ||
Revision as of 01:47, 26 April 2024
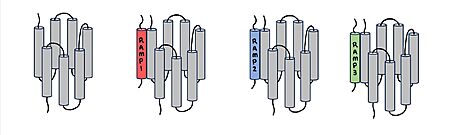
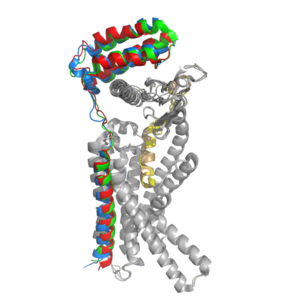
Amylin Receptor (AMYR)
| |||||||||||
References
- ↑ Cao J, Belousoff MJ, Liang YL, Johnson RM, Josephs TM, Fletcher MM, Christopoulos A, Hay DL, Danev R, Wootten D, Sexton PM. A structural basis for amylin receptor phenotype. Science. 2022 Mar 25;375(6587):eabm9609. PMID:35324283 doi:10.1126/science.abm9609
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Hay DL, Chen S, Lutz TA, Parkes DG, Roth JD. Amylin: Pharmacology, Physiology, and Clinical Potential. Pharmacol Rev. 2015 Jul;67(3):564-600. PMID:26071095 doi:10.1124/pr.115.010629
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 Cao J, Belousoff MJ, Liang YL, Johnson RM, Josephs TM, Fletcher MM, Christopoulos A, Hay DL, Danev R, Wootten D, Sexton PM. A structural basis for amylin receptor phenotype. Science. 2022 Mar 25;375(6587):eabm9609. PMID:35324283 doi:10.1126/science.abm9609
- ↑ 4.0 4.1 Press, M., Jung, T., Konig, J., Grune, T., & Hohn, A. (2019) Protein aggregates and proteostasis in aging: Amylin and β-cell function. Mechanisms of Ageing and Development. 3, 46-54. DOI:10.1016/j.mad.2018.03.010
- ↑ Mathiesen DS, Lund A, Vilsbøll T, Knop FK, Bagger JI. Amylin and Calcitonin: Potential Therapeutic Strategies to Reduce Body Weight and Liver Fat. Front Endocrinol (Lausanne). 2021 Jan 8;11:617400. PMID:33488526 doi:10.3389/fendo.2020.617400
- ↑ Thapa, G., Kumari, A., Dasgupta, D., Bandyopadhy, S., Sarkar, N., Roy, K., Karunakaran, G., Kazmi, I., Karmakar, S., & Chakraborty, M. (2023). Chapter 5- Insight into the mechanism of action of anti-diabetic drugs. How Synthetic Drugs Work. 95-122. DOI:10.1016/B978-0-323-99855-0.00005-1
- ↑ Grizzanti J, Corrigan R, Casadesus G. Neuroprotective Effects of Amylin Analogues on Alzheimer's Disease Pathogenesis and Cognition. J Alzheimers Dis. 2018;66(1):11-23. PMID:30282360 doi:10.3233/JAD-180433