Introduction
Research Question
What is the function and qualities (location and optimal conditions) of our assigned unknown protein, 2QRU?
Problem Relevance
The function of this protein is not known past the fact that it is in the super family alpha/beta hydrolase. Knowing the function of this protein could be helpful since knowing a protein’s function allows for extensive classification of said protein.
Research Significance
Identifying the structure of the unknown protein would reveal its function which could be used in many different ways. By connecting the protein’s function to symptoms and mechanisms, it could be used as a target for treatment of various diseases.
Hypothesis
Based on the super family classification, protein 2QRU is a type of hydrolase. Running experiments to test the protein under three different pH conditions based on the three most common locations of hydrolases (digestive system, neurons, and muscle cells) will reveal the activity of the protein in these different pH’s and therefore could suggest where the protein is found and consequently its function.
How Experimental Data Will Answer the Hypothesis
o The different computer modeling strategies used such as Chimera, SPRITE, Dali, BLAST, InterPRO, and SwissDock allowed visualization of the protein’s structure as a whole
and at the amino acid level, showed us different alignments and active sites, compared it to other proteins for function analysis, showed different substrate interactions and much
more which we will dive into in the experimental section.
o PAGE Gel told us whether the purification process worked or not and which sample to use when we tested for enzyme activity based on the strongest band.
o Bradford assay provided a concentration of our samples and determined which sample would be used for further testing.
o UV-Vis provided protein activity in different pH’s (more activity meant more absorbance from color change and therefore possible conclusions that the
enzyme functioned in an area of the body at that pH).
Materials & Methods
Computer Modeling Strategies
o Chimera: allowed visualization of the protein; was used to compare conformation, identity, and conservation of amino acid side chains; was used instead of Dali (Dali didn’t analyze side chains); visualized results from SPRITE in a different way.
o SPRITE: evaluated local alignments- only a small part of the protein; identified active sites; was used to search for configurations of amino acid side chains that have a similar structure to those of known enzyme active sites.
o Dali: aligned the entire 3D protein (global alignment), matched structurally similar proteins rather than sequence similarities like BLAST. Dali is more limited than BLAST since it can only work with protein structures as a whole (there are fewer known structures than known sequences); only had matches based on the backbone of the protein, did not involve amino acid side chains or side chains that would show functionality of the enzyme.
o BLAST: sequence searching that only matched similar amino acid sequences rather than the whole protein; Looked at the sequence in sections, found matching overlaps with sequences in the database, scored the matches based on similarity, and provided a list of matches.
o InterPro: matched similar sequences rather than the whole protein; matched the unknown protein to a family which gave clues about its function, where it is, and general information about qualities that it might share with other proteins in that family.
o SwissDock: computationally predicted how substrates would interact and bind with the active site or allosteric sites; Tested various ligands and provided binding energies.
Key Information about Chemicals, Equipment, & Instrumentation
o Escherichia coli (E. coli) strain pLann
o Buffers (Sodium Phosphate buffer, Tris-HCl, Re-Suspension Buffer, Cell Lysis Buffer, 1X Wash Buffer, 1X Elution Buffer, 10X SDS-PAGE Buffer, Coomassie Blue Stain, and Destain) were prepared by classmates with appropriate techniques and all materials were stored at necessary temperatures.
o Equipment used: computer modeling systems, Bradford Assay, PAGE, sonicator (Qsonica Sonicators), centrifuge (SORVALL RC 5C Plus), UV-Vis (Olis 8453), freezer (Glacier, -86°C, ULTRALOW TEMPERATURE FREEZER)
o Protein samples were kept on ice to avoid denaturing due to temperature changes.
o Glassware was cleaned, all equipment was properly prepared prior to the start of the lab, and contamination was reduced through the use of disposable cuvettes and pipette tips.
Results
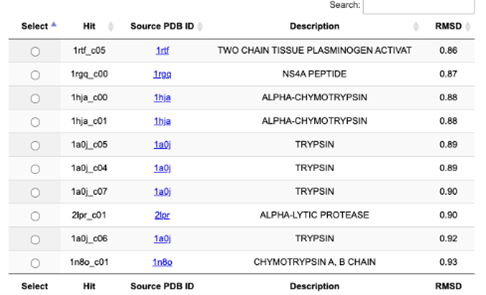
Figure 1: List of hits of 2QRU from SPRITE (10 of 200 entries shown here). The first column tells what hit each row shows and the second column has the source PDB ID that the program uses to identify each protein in the database. The third column gives a description of the protein and the final column shows the RMSD value of that protein when compared to the search protein of 2QRU.
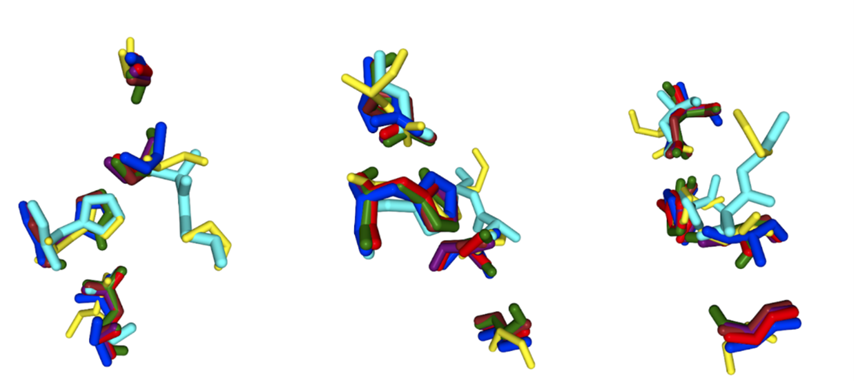
Figure 2: Alignment of 2QRU with 6 different motifs (A 33 GLY, A 102 SER, A 219 ASP, A 247 HIS; A 102 SER, A 130 GLY, A 219 ASP, A 247 HIS; A 102 SER, A 104 GLY, A 219 ASP, A 247 HIS; A 34 GLY, A 102 SER, A 219 ASP, A 247 HIS; A 102 SER, A 219 ASP, A 247 HIS; A 104 GLY, A 219 ASP, A 247 HIS) from 3 different views in SPRITE. Alignment was generated from the “Full Details” and the “Superposed motifs” function was utilized such that all proteins are visible.
Figure 3: Protein #0 with colors by element. This image was generated in CHIMERA to observe the shape of the protein 2QRU and later is compared to other similar proteins given by SPRITE to determine their relative similarities.
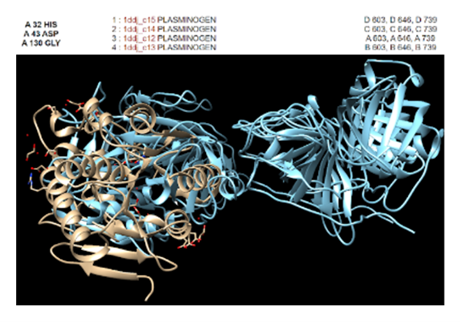
Figure 4: In Chimera, protein 2QRU matched the most with enzymes such as trypsins, proteases, and chymotrypsins. However, the best RMSD value of 2.328 Angstroms was found to be with this plasminogen.
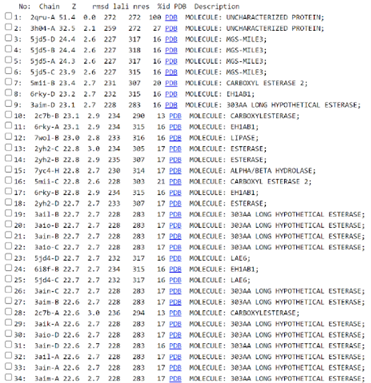
Figure 5: Results from 2QRU search in Dali and comparison to the full PDB. The first column shows the chain name, the second shows the z-value, and the third shows the RMSD value. Next is the lali value, which tells the number of equivalent residues and the total number of residues. Then the %id to identify what the result is in the program and finally a description of what the protein result is.
>2QRU_1|Chain A|Uncharacterized protein|Enterococcus faecalis (226185) :
SNAHLKNNQTLANGATVTIYPTTTEPTNYVVYLHGGGMIYGTKSDLPEELKELFTSNGYTVLALDYLLAPNTKIDHILRTLTETFQLLNEEIIQNQSFGLCGRSAGGYLMLQLTKQLQTLNLTPQFLVNFYGYTDLEFIKEPRKLLKQAISAKEIAAIDQTKPVWDDPFLSRYLLYHYSIQQALLPHFYGLPENGDWSAYALSDETLKTFPPCFSTASSSDEEVPFRYSKKIGRTIPESTFKAVYYLEHDFLKQTKDPSVITLFEQLDSWLKER
Figure 6: RCSB Website FASTA sequence of 2QRU. Decreasing word size from 5→2 did not change number of results in BLAST.
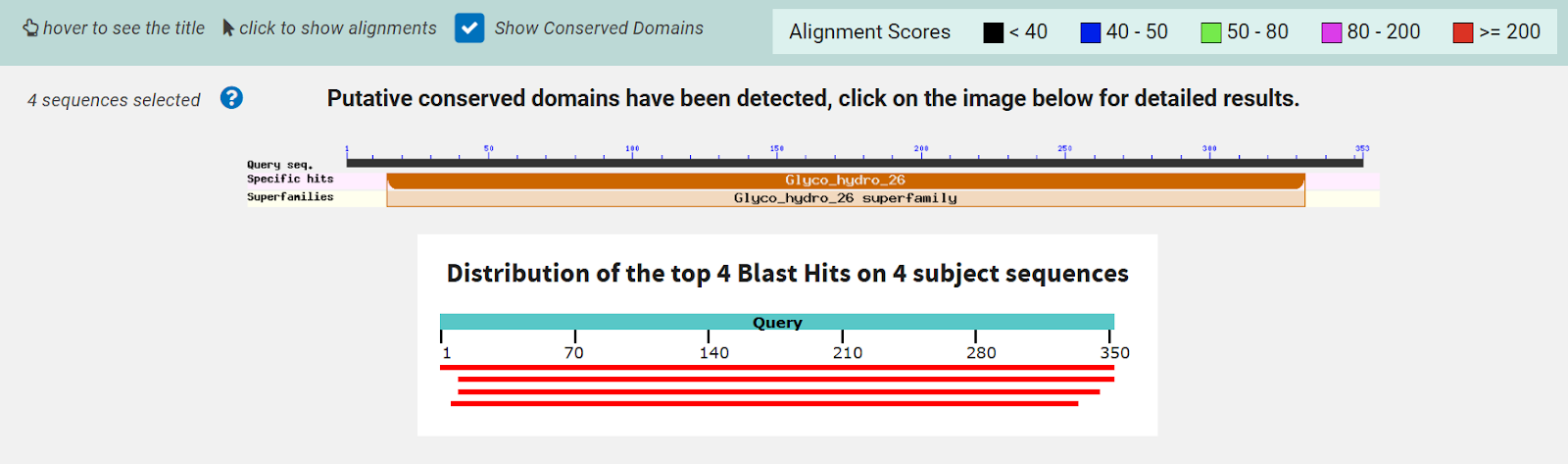
Figure 7: RCSB PDB Result Page for 2QRU. This page shows an overview of information about protein 2QRU including several classifications.
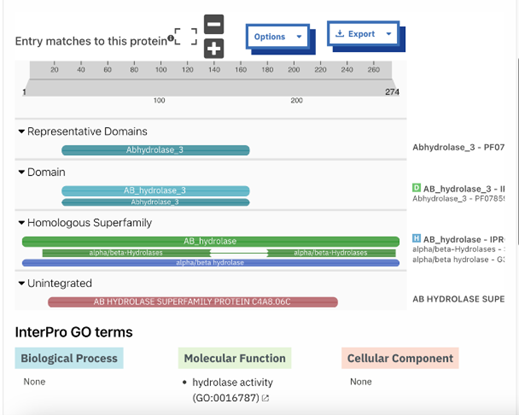
Figure 8: InterPRO results 2QRU. This shows the main page of InterPRO when searching 2QRU and it includes information such as the possible protein family membership (although there was no match for this particular protein). It also shows several domains that are similar matches to 2QRU, all of which are types of hydrolases, suggesting that 2QRU is a type of hydrolase as well. It gives the results for the biological process (none), the molecular function (hydrolase), and the cellular component (none). Some information is missing in this page which is what our research aimed to help fill in.
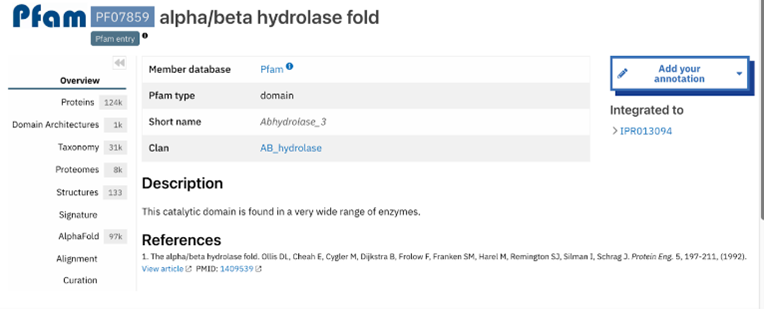
Figure 9: Abhydrolase_3 - PF07859: alpha/beta hydrolase fold profile. This result was found by selecting the domain from the InterPro overview page shown in figure 8. This gives more information about that domain specifically. The information includes several classifications as well as options on the side bar to investigate the result further.
Figure 10: AB_hydrolase_3 - IPR013094: alpha/beta hydrolase fold-3 profile. This result was found by selecting another one of the domains from the InterPro overview page shown in figure 8 and this gives more information about that domain specifically. The information includes several classifications as well as options on the side bar to investigate the result further.
Figure 11: AB_hydrolase - IPR029058: alpha.beta hydrolase fold profile. This result was found by selecting another one of the domains from the InterPro overview page shown in figure 8 and this gives more information about that domain specifically. The information includes several classifications as well as options on the side bar to investigate the result further.
Figure 12: Structure results of IPR029058- alpha/beta hydrolase fold. This was found by selecting the side bar option of structure in Interpro. This provided information about the structural qualities of proteins in that domain.
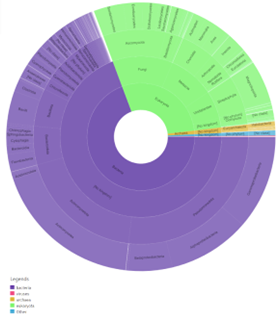
Figure 13: Taxonomy results of IPR029058- alpha/beta hydrolase fold. This was found by selecting the side bar options of taxonomy in Interpro. This provided information about the taxonomic background of proteins in that domain. (KEY: purple- bacteria, pink- viruses, yellow- archaea, green- eukaryote, blue- other).
Discussion
Since the unknown enzyme had a recognizable activity with the common hydrolase substrate, p-nitrophenyl acetate, it is likely that 2QRU is indeed a hydrolase. When unknown protein 2QRU was tested in a pH of ~2, the enzyme completely dissociated from solution indicating it can in no way function in that acidic of an environment in the body. Since the protein worked best in the pH around neutral, it is likely that the enzyme functions as a hydrolase in either neuronal or muscle cells.
Accuracy & Precision of Results
Our results are partially accurate as we tested our protein with a general substrate for hydrolases. Although not directly associated with our enzymes exact active site, the substrate we used, p-nitrophenyl acetate (PNPA), does generally work with hydrolases as a whole. In terms of our results from the computer models and directly from the equipment used, those results are accurate as the databases are reliable and the equipment was calibrated and used correctly.
Our results may be precise, but we would need further testing and repeats of our results to determine whether they are statistically different or precise enough to be the same. We only ran our experiments and each of our pH levels once, so we cannot confidently confirm precision.
Future Experiments
Using a substrate that has been shown in the computer modeling to work more directly with the expected active site of our protein can give more specific insight into the type of hydrolase that 2QRU may be.
When testing with UV-Vis, taking readings at closer time points can allow for more precise results.
Repeating the experiment multiple times to increase the reliability of the results.
Conclusion
Overall, the hypothesis that the protein 2QRU is a type of hydrolase was determined through testing with a general hydrolase-recognized substrate p-nitrophenyl acetate. After testing at three different pH environments, the best pH level for this enzyme seemed to be at a more neutral, between 6.4 and 7.2. After computational testing, the best matched substrates are suspected to be 4-nitroacetanilide and alanine-p-nitroanilide. Enzyme activity testing has not been completed thus far with these substrates, but that work could indicate what specific substrates would be best matched with the enzyme providing more specifics into the structure and function of 2QRU.
References
The BASIL Biochemistry Curriculum. basilbiochem.org (n.d.). Ashley Ringer McDonald1 , Herbert J. Bernstein2, S. Colette Daubner3, Jonathan D. Dattelbaum4, Anya Goodman1, Bonnie L. Hall5, Stefan M. Irby6, Julia R. Koeppe7, Jeffrey L. Mills2, Stephen A. Mills8, Suzanne F. O’Handley2, Michael Pikaart9, Rebecca Roberts10, Arthur Sikora11, Paul A. Craig2










