We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Nathan Marohn/Sandbox 2
From Proteopedia
(Difference between revisions)
| Line 25: | Line 25: | ||
=== Extracellular Domain === | === Extracellular Domain === | ||
| - | In the extracellular domain, extensive pi-stacking occurs between <scene name='10/1043643/Gip_ecd_pi_stacking/1'>GIP to GIP-R</scene> and <scene name='10/1037512/Tirz_gipr_ecd_pi_stacking/5'>Tirzepatide to GIP-R</scene>. Aromatic residues F22 and W25 are conserved on Tirzepatide to maintain strong hydrophobic | + | In the extracellular domain, extensive pi-stacking occurs between <scene name='10/1043643/Gip_ecd_pi_stacking/1'>GIP to GIP-R</scene> and <scene name='10/1037512/Tirz_gipr_ecd_pi_stacking/5'>Tirzepatide to GIP-R</scene>. Aromatic residues F22 and W25 are conserved on Tirzepatide to maintain strong hydrophobic interactions with Y36 and W39 on GIP-R. However, Tirzepatide contains a key H18A mutation<ref name="Sun"/> that increases the affinity of the ligand compared to GIP. Although this mutation inactivates a hydrogen bond previously formed by H18, it increases Tirzepatide’s affinity for the GLP-R by reducing steric clashing and allowing the ligand to bind deeper into the receptor. |
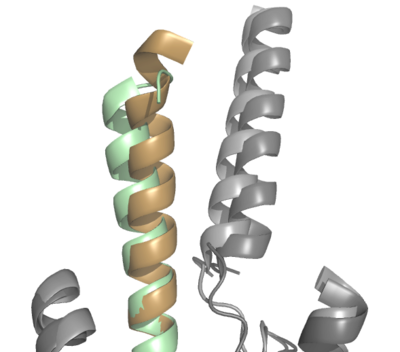
=== GIP-R Bound to GIP vs. Tirzepatide === | === GIP-R Bound to GIP vs. Tirzepatide === | ||
| - | The natural GIP ligand (tan with dark gray GIP-R) and the synthetic Tirzepatide ligand (green with light gray GIP-R) do not bind to the receptor analogously. The modifications applied to Tirzepatide caused a shift in the orientation of ligand binding (Fig. 2). Specifically, the H18A and I7T mutations shaped the synthetic ligand to bind differently than the natural ligand, in which the extracellular domain of GIP-R had to adjust to fit the new binding angle. Ultimately, the binding configuration induced by Tirzepatide tethers the ligand deeper into the receptor transmembrane domain, increasing its affinity for the receptor (Fig. 2). | + | The natural GIP ligand (tan with dark gray GIP-R) and the synthetic Tirzepatide ligand (green with light gray GIP-R) do not bind to the receptor analogously. The modifications applied to Tirzepatide caused a shift in the orientation of ligand binding (Fig. 2). Specifically, the H18A and I7T mutations shaped the synthetic ligand to bind differently than the natural ligand, in which the extracellular domain of GIP-R had to adjust to fit the new binding angle. Ultimately, the binding configuration induced by Tirzepatide tethers the ligand deeper into the receptor transmembrane domain, increasing its affinity for the receptor (Fig. 2). [[Image:ProtoOverlay1.png|400 px|center|thumb|Figure 2]] |
== Future Implications == | == Future Implications == | ||
Revision as of 14:51, 26 April 2024
Glucose-dependent insulinotropic polypeptide receptor (GIP-R)
| |||||||||||
References
- ↑ 1.0 1.1 Dalle S, Quoyer J, Varin E, Costes S. Roles and regulation of the transcription factor CREB in pancreatic β -cells. Curr Mol Pharmacol. 2011 Nov;4(3):187-95. PMID:21488836 doi:10.2174/1874467211104030187
- ↑ 2.0 2.1 2.2 2.3 2.4 Sun B, Willard FS, Feng D, Alsina-Fernandez J, Chen Q, Vieth M, Ho JD, Showalter AD, Stutsman C, Ding L, Suter TM, Dunbar JD, Carpenter JW, Mohammed FA, Aihara E, Brown RA, Bueno AB, Emmerson PJ, Moyers JS, Kobilka TS, Coghlan MP, Kobilka BK, Sloop KW. Structural determinants of dual incretin receptor agonism by tirzepatide. Proc Natl Acad Sci U S A. 2022 Mar 29;119(13):e2116506119. PMID:35333651 doi:10.1073/pnas.2116506119
- ↑ 3.0 3.1 3.2 Zhao F, Zhou Q, Cong Z, Hang K, Zou X, Zhang C, Chen Y, Dai A, Liang A, Ming Q, Wang M, Chen LN, Xu P, Chang R, Feng W, Xia T, Zhang Y, Wu B, Yang D, Zhao L, Xu HE, Wang MW. Structural insights into multiplexed pharmacological actions of tirzepatide and peptide 20 at the GIP, GLP-1 or glucagon receptors. Nat Commun. 2022 Feb 25;13(1):1057. PMID:35217653 doi:10.1038/s41467-022-28683-0
- ↑ Mayendraraj A, Rosenkilde MM, Gasbjerg LS. GLP-1 and GIP receptor signaling in beta cells interactions and co-stimulation. Peptides. 2022 May;151:170749. PMID:35065096 doi:10.1016/j.peptides.2022.170749
- ↑ Seino Y, Fukushima M, Yabe D. GIP and GLP-1, the two incretin hormones: Similarities and differences. J Diabetes Investig. 2010 Apr 22;1(1-2):8-23. PMID:24843404 doi:10.1111/j.2040-1124.2010.00022.x
- ↑ Yaqub T, Tikhonova IG, Lättig J, Magnan R, Laval M, Escrieut C, Boulègue C, Hewage C, Fourmy D. Identification of determinants of glucose-dependent insulinotropic polypeptide receptor that interact with N-terminal biologically active region of the natural ligand. Mol Pharmacol. 2010 Apr;77(4):547-58. PMID:20061446 doi:10.1124/mol.109.060111