This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
User:SaraKathryn Kalkhoff/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
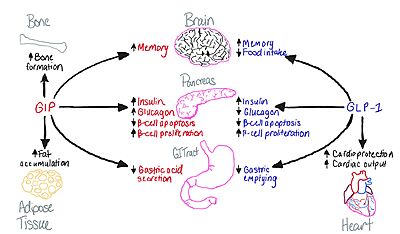
[[Image:Gip glp.jpeg|400 px|right|thumb|Figure 1: Distinction of functions between the GIP and GLP-1 receptors.]] | [[Image:Gip glp.jpeg|400 px|right|thumb|Figure 1: Distinction of functions between the GIP and GLP-1 receptors.]] | ||
===History=== | ===History=== | ||
| - | + | The main receptor, originally named Gastric Inhibiory Polypeptide, was found in 1902 and shown to have impacts on secretory pathways present within the cell. It wasn't until 1984, when the receptor was found and have many of the functions it has today. This is able to play into discovering the Type 2 Diabetes medication, Mounjaro (tirzepatide), | |
== Function == | == Function == | ||
The GIP receptor helps facilitate movement of glucose within a cell. <ref name='Sun'>PMID:35333651</ref>. It has a natural ligand that is 42 residues and helps kickstart the GIP-R into firing, as a transporter for glucose in and out of the cell. Once the levels become too high, the ligand will send this transporter back down into the cell and not have anymore glucose activate. It will also cause the insulin pathway to start to help signal that there is too much glucose in the body. As mentioned above, many issues that arise with diabetes can affect the rate of transport within these transporters for the cell. | The GIP receptor helps facilitate movement of glucose within a cell. <ref name='Sun'>PMID:35333651</ref>. It has a natural ligand that is 42 residues and helps kickstart the GIP-R into firing, as a transporter for glucose in and out of the cell. Once the levels become too high, the ligand will send this transporter back down into the cell and not have anymore glucose activate. It will also cause the insulin pathway to start to help signal that there is too much glucose in the body. As mentioned above, many issues that arise with diabetes can affect the rate of transport within these transporters for the cell. | ||
Revision as of 21:00, 27 April 2024
| |||||||||||