Introduction
Glucose-dependent insulinotropic polypeptide is a gastric inhibitory polypeptide, composed of 42 amino acids. It is categorized as an incretin, which is a gut-based hormone that is secreted upon the ingestion of food. It is found in K cells in the upper small intestines of humans.[1]
History
The idea of incretin, and subsequently GIP, began in 1902 after the discovery of secretin by Bayliss and Starling. Inspired by their discovery, Moore et al. hypothesized that there must be some kind of gut hormone that regulates the endocrine pancreas. His experiments showed that gut extracts in patients with diabetes have reduced amounts of sugars in their urine as a result of endocrine stimulation. In 1929, a French scientist named La Barre was able to purify incretin (INtestine seCREtion INsulin) from gut extracts. The idea of incretin slowly lost attention until the 1960s when radioimmunoassay became available to measure incretin levels. GIP and GLP-1 (glucagon-like peptide-1) were the two gut based hormones that were proven to act as incretins. GIP was found to be located in the K cells of the upper small intestines in humans. At first, it was titled a gastric inhibitory polypeptide because of its ability to inhibit gastric acid secretion after being isolating from porcine intestines. Later, they discovered that when GIP was administered in healthy volunteers, it stimulated insulin secretion by acting directly on pancreatic islets. As a result of these discoveries, they renamed it glucose-dependent insulinotropic polypeptide, becoming the first known incretin.[2]
Structure Overview
G-proteins
is a G-coupled receptor GPCR. It is a 7 helical transmembrane domain with the ligand in dark blue and the helices in light blue. It contains alpha, beta, and gamma located in the intracellular domain. The G alpha subunit makes interactions with the beta and gamma subunits that when bound to ATP, activates the G alpha protein and is released by the other subunits to bind to target, i.e. adenylyl cycle in order to activate the process of insulin secretion.[2]
Function
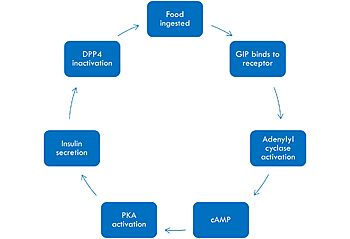
Mechanism of GIP activation and deactivation.
GIP is a vital hormone for the daily function of humans. It is involved in increased bone formation as well as increased lipogenesis, which is the conversion and storage of fats as triglycerides in adipocyte tissues. In the brain, GIP acts increase brain cell proliferation, which is very important for early stage development in children. In the pancreas, GIP acts to increase insulin production, increase glucagon levels, increase beta cell proliferation as well as decrease beta cell apoptosis levels. In the GI tract, this hormone acts to decrease gastric acid secretion.[2]
Mechanism
The mechanism of GIP begins with the ingestion of food. This stimulates GIP to bind to its receptor. Once bound, the activated G alpha protein moves laterally in the membrane in order to activate its target, adenylyl cyclase. Once adenylyl cyclase is activated, it catalyzes the formation of cyclic AMP (cAMP). This molecule activates Protein Kinase A (PKA), which signals the secretin of insulin. After a few minutes of active signaling, this hormone is recognized and inactivated by a peptidase called DPP-4 (dipeptidyl peptidase-4) by cleaving the first two amino acids.[1]
Diseases
Malfunction of the GIP protein can result in some serious life-threatening diseases. Overexpression of GIP can result in obesity by promoting fat disposition in adipocyte tissues as well as an inability to regulate food intake and control appetite. Underexpression of GIP can result in diabetes, a disease associated with the lack of insulin biosynthesis, which is vital for energy production.
Tirzepatide
One of the most promising treatments to GIP malfunction is , also known as Mounjaro, created by Eli Lily. It is an antidiabetic as well as weight loss drug. The structure of Tirzepatide functions as an incretin receptor agonist to GIP by activating GIP's receptor and producing insulin.
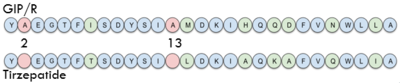
Sequence comparison of the first 28 residues of GIP and Tirzepatide.
Structural highlights
Binding/Active Site of GIPR with GIP
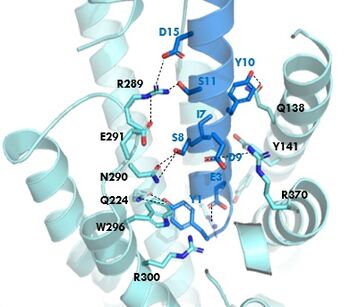
GIPR (light blue) and GIP (dark blue) residue interactions
The of GIP with the GIP receptor (GIPR) is where the N-term of GIP binds with the transmembrane domain of the GIPR. The first interaction formed with GIPR and the N-term of GIP is a hydrogen bond between Tyrosine 1 (Y1) and Glutamine 224 (Q224) to activate the G-protein to start sending signals to the cell.
Many other within the binding site are forming hydrogen bonds and hydrophobic interactions between the ligand and the receptor. The N-term binds more strongly than the C-term and there are many different residues contributing to this, including, Tyrosine 1 (Y1) and Tryptophan (W296) are forming aromatic interactions. Serine 8 (S8) and Asparagine 290 (N290) are forming two hydrogen bonds with each other. Aspartate 9 (D9) is forming another hydrogen bond with Arginine 370 (R370)[3]. All of these interactions are responsible for the binding affinity, the strength of the attraction, between the two proteins. These hydrogen bonds lead to the activation of cell signaling and when this binding is somehow disrupted, that is what causes different diseases like diabetes.
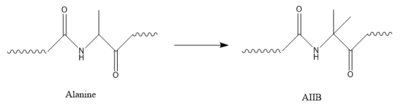
Structure of alanine converted to AIB.
Relevance
In GIP, the residue 2 is an but in Tirzepatide, the residue changes to
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.




