We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Mandy Bechman/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 30: | Line 30: | ||
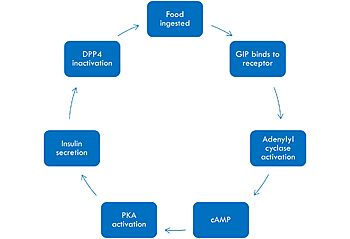
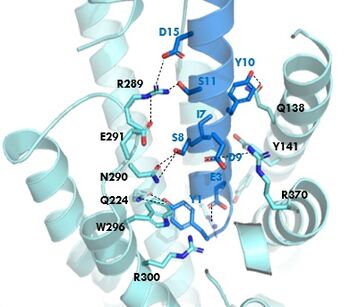
The <scene name='10/1038815/Overview/7'>binding site</scene> of GIP with the GIP receptor (GIPR) is where the N-term of GIP binds with the transmembrane domain of the GIPR. The first interaction formed with GIPR and the N-term of GIP is a hydrogen bond between Tyrosine 1 (Y1) and Glutamine 224 (Q224) to activate the G-protein to start sending signals to the cell. | The <scene name='10/1038815/Overview/7'>binding site</scene> of GIP with the GIP receptor (GIPR) is where the N-term of GIP binds with the transmembrane domain of the GIPR. The first interaction formed with GIPR and the N-term of GIP is a hydrogen bond between Tyrosine 1 (Y1) and Glutamine 224 (Q224) to activate the G-protein to start sending signals to the cell. | ||
Many other <scene name='10/1038815/Active_site/3'>residues</scene> within the binding site are forming hydrogen bonds and hydrophobic interactions between the ligand and the receptor. The N-term binds more strongly than the C-term and there are many different residues contributing to this, including, Tyrosine 1 (Y1) and Tryptophan (W296) are forming aromatic interactions. Serine 8 (S8) and Asparagine 290 (N290) are forming two hydrogen bonds with each other. Aspartate 9 (D9) is forming another hydrogen bond with Arginine 370 (R370)<ref name="Sun"/>. All of these interactions are responsible for the binding affinity, the strength of the attraction, between the two proteins. These hydrogen bonds lead to the activation of cell signaling and when this binding is somehow disrupted, that is what causes different diseases like diabetes. | Many other <scene name='10/1038815/Active_site/3'>residues</scene> within the binding site are forming hydrogen bonds and hydrophobic interactions between the ligand and the receptor. The N-term binds more strongly than the C-term and there are many different residues contributing to this, including, Tyrosine 1 (Y1) and Tryptophan (W296) are forming aromatic interactions. Serine 8 (S8) and Asparagine 290 (N290) are forming two hydrogen bonds with each other. Aspartate 9 (D9) is forming another hydrogen bond with Arginine 370 (R370)<ref name="Sun"/>. All of these interactions are responsible for the binding affinity, the strength of the attraction, between the two proteins. These hydrogen bonds lead to the activation of cell signaling and when this binding is somehow disrupted, that is what causes different diseases like diabetes. | ||
| + | === Binding/Active Site of GIPR with Tirzepatide === | ||
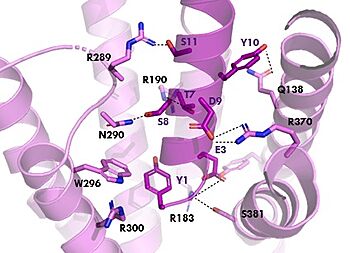
| + | [[Image:TZ_hydrogen_bonds.jpg|350 px|right|thumb|GIPR (pink) and Tirzepatide (dark pink) residue interactions]] | ||
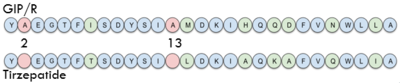
| + | The <scene name='10/1038815/Tirzepatide_active_site/4'>binding site</scene> with Tirzepatide is the same as GIP with the N-term binding to the transmembrane domain and activating cellular signaling.The <scene name='10/1038815/Tirzepatide_residues/7'>residues</scene> are fairly similar just in a different conformations, which is allowing for more hydrogen bonding. In Tirzepatide, the most noticeable conformational change is the Tyrosine 1 (Y1) residue. It is now facing up towards Arginine 190 (R190) where they can now form a new hydrogen bond that was not present in GIP. The more hydrogen bonding there is between molecules, the stronger the binding between the two molecules will be. This can lead to the conclusion that GIPR has a higher binding affinity for Tirzepatide than GIP itself<ref name="Sun"/>. | ||
| + | |||
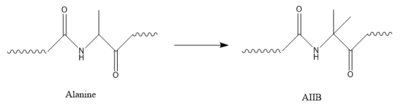
[[Image:AlatoAIB.png|400 px|left|thumb|Structure of alanine converted to AIB.]] | [[Image:AlatoAIB.png|400 px|left|thumb|Structure of alanine converted to AIB.]] | ||
| Line 44: | Line 48: | ||
<ref name="Seino">PMID:24843404</ref>. | <ref name="Seino">PMID:24843404</ref>. | ||
<ref name="Mayendraraj">PMID:35065096</ref>. | <ref name="Mayendraraj">PMID:35065096</ref>. | ||
| + | <ref name="Sun">PMID:35333651</ref> | ||
<references/> | <references/> | ||
Revision as of 22:23, 28 April 2024
H. sapiens Glucose-dependent Insulinotropic Polypeptide
| |||||||||||
References
- ↑ 1.0 1.1 1.2 Mayendraraj A, Rosenkilde MM, Gasbjerg LS. GLP-1 and GIP receptor signaling in beta cells interactions and co-stimulation. Peptides. 2022 May;151:170749. PMID:35065096 doi:10.1016/j.peptides.2022.170749
- ↑ 2.0 2.1 2.2 2.3 Seino Y, Fukushima M, Yabe D. GIP and GLP-1, the two incretin hormones: Similarities and differences. J Diabetes Investig. 2010 Apr 22;1(1-2):8-23. PMID:24843404 doi:10.1111/j.2040-1124.2010.00022.x
- ↑ 3.0 3.1 3.2 Sun B, Willard FS, Feng D, Alsina-Fernandez J, Chen Q, Vieth M, Ho JD, Showalter AD, Stutsman C, Ding L, Suter TM, Dunbar JD, Carpenter JW, Mohammed FA, Aihara E, Brown RA, Bueno AB, Emmerson PJ, Moyers JS, Kobilka TS, Coghlan MP, Kobilka BK, Sloop KW. Structural determinants of dual incretin receptor agonism by tirzepatide. Proc Natl Acad Sci U S A. 2022 Mar 29;119(13):e2116506119. PMID:35333651 doi:10.1073/pnas.2116506119
Student Contributors
Mandy M. Bechman Chloe A. Tucker