We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox324
From Proteopedia
(Difference between revisions)
| Line 21: | Line 21: | ||
'''Figure 1: '''Results from SPRITE of top 10 matching proteins With 4DIU based on amino acid matches, RMSD, and ASSAM. | '''Figure 1: '''Results from SPRITE of top 10 matching proteins With 4DIU based on amino acid matches, RMSD, and ASSAM. | ||
| + | |||
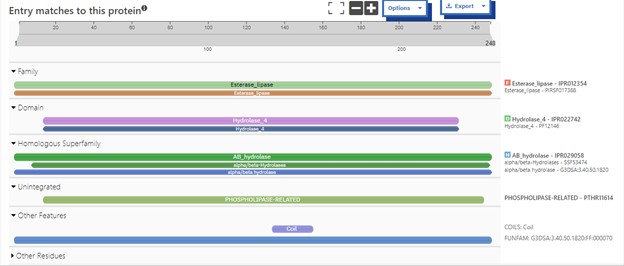
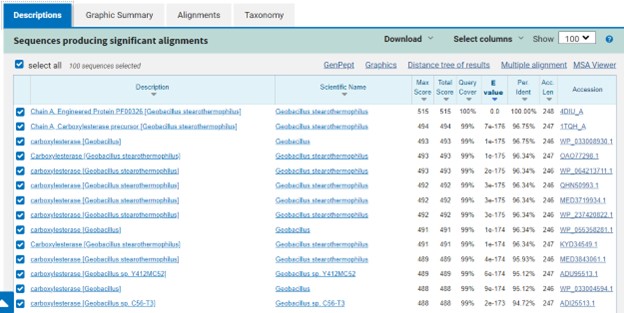
Additional evidence is provided by InteroPro which provided more information about the classification of the protein. As well, the BLAST search gave results of matching proteins and the specific functions of the proteins. All results again matched what was suggested by Sprite and Blast, further solidifying that 4DIU is an esterase with alpha/beta-hydrolase activity. | Additional evidence is provided by InteroPro which provided more information about the classification of the protein. As well, the BLAST search gave results of matching proteins and the specific functions of the proteins. All results again matched what was suggested by Sprite and Blast, further solidifying that 4DIU is an esterase with alpha/beta-hydrolase activity. | ||
| - | [[Image:InterPro.jpg]][[Image:Blast.jpg]] | + | [[Image:InterPro.jpg]] |
| + | '''Figure 2: '''Interpro Scan results for 4DIU displaying proposed characterization | ||
| + | [[Image:Blast.jpg]] | ||
| + | '''Figure 3: '''Blast search for protein sequences matching 4DIU | ||
| + | |||
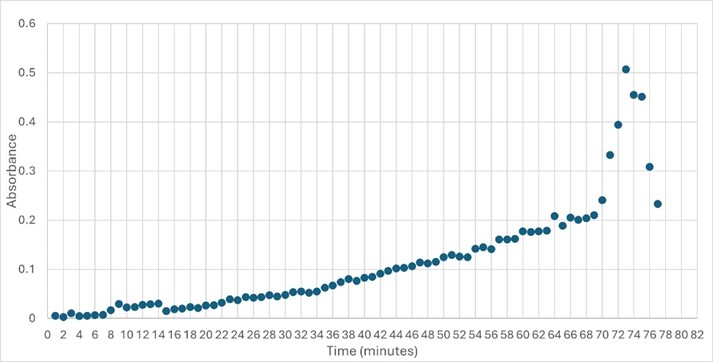
SwissDock and Chimera allowed for protein-ligand docking studies. Many of the highlighted interactions were ester-containing ligands and others were susceptible to hydrolysis. The hydrolase activity of the protein needed to be tested with a substrate known to work with hydrolases. This substrate is p-nitrophenyl acetate PNP when hydrolyzed produces a yellow colored product. The yellow product will lead to the solution absorbing more at 405nm. <ref>Zhang, S.; Sun, W.; Xu, L.; Zheng, X.; Chu, X.; Tian, J.; Wu, N.; Fan, Y. Identification of the Para-Nitrophenol Catabolic Pathway, and Characterization of Three Enzymes Involved in the Hydroquinone Pathway, in Pseudomonas Sp. 1-7. BMC Microbiology 2012, 12 (1). https://doi.org/10.1186/1471-2180-12-27. | SwissDock and Chimera allowed for protein-ligand docking studies. Many of the highlighted interactions were ester-containing ligands and others were susceptible to hydrolysis. The hydrolase activity of the protein needed to be tested with a substrate known to work with hydrolases. This substrate is p-nitrophenyl acetate PNP when hydrolyzed produces a yellow colored product. The yellow product will lead to the solution absorbing more at 405nm. <ref>Zhang, S.; Sun, W.; Xu, L.; Zheng, X.; Chu, X.; Tian, J.; Wu, N.; Fan, Y. Identification of the Para-Nitrophenol Catabolic Pathway, and Characterization of Three Enzymes Involved in the Hydroquinone Pathway, in Pseudomonas Sp. 1-7. BMC Microbiology 2012, 12 (1). https://doi.org/10.1186/1471-2180-12-27. | ||
| Line 29: | Line 34: | ||
</ref> | </ref> | ||
[[Image:Enzymeactivity.jpeg]] | [[Image:Enzymeactivity.jpeg]] | ||
| + | '''Figure 3: ''' Trial 2 of 4DIU enzymatic activity measured over time via change in absorbance at pH 6 | ||
== Structural highlights of 4DIU == | == Structural highlights of 4DIU == | ||
Revision as of 23:53, 28 April 2024
| |||||||||||
References
- ↑ Zhang, S.; Sun, W.; Xu, L.; Zheng, X.; Chu, X.; Tian, J.; Wu, N.; Fan, Y. Identification of the Para-Nitrophenol Catabolic Pathway, and Characterization of Three Enzymes Involved in the Hydroquinone Pathway, in Pseudomonas Sp. 1-7. BMC Microbiology 2012, 12 (1). https://doi.org/10.1186/1471-2180-12-27.
- ↑ Vázquez-Mayorga, E.; Díaz-Sánchez, Á.; Dagda, R.; Domínguez-Solís, C.; Dagda, R.; Coronado-Ramírez, C.; Martínez-Martínez, A. Novel Redox-Dependent Esterase Activity (EC 3.1.1.2) for DJ-1: Implications for Parkinson’s Disease. International Journal of Molecular Sciences 2016, 17 (8), 1346. https://doi.org/10.3390/ijms17081346.
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644