We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Karisma Moll/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 40: | Line 40: | ||
Diabetes mellitus is a metabolic disorder, characterized by hyperglycemia, caused by irregularity in insulin secretion, insulin action, or a combination of both. [https://en.wikipedia.org/wiki/Type_1_diabetes Type 1 diabetes mellitus] (T1DM) is characterized by the autoimmune destruction of pancreatic beta-cells, leading to diabetic ketoacidosis. [https://en.wikipedia.org/wiki/Type_2_diabetes Type 2 diabetes mellitus] (T2DM) is characterized by insulin resistance and subsequent deficiency. <ref name="Banday">PMID:33437689</ref> | Diabetes mellitus is a metabolic disorder, characterized by hyperglycemia, caused by irregularity in insulin secretion, insulin action, or a combination of both. [https://en.wikipedia.org/wiki/Type_1_diabetes Type 1 diabetes mellitus] (T1DM) is characterized by the autoimmune destruction of pancreatic beta-cells, leading to diabetic ketoacidosis. [https://en.wikipedia.org/wiki/Type_2_diabetes Type 2 diabetes mellitus] (T2DM) is characterized by insulin resistance and subsequent deficiency. <ref name="Banday">PMID:33437689</ref> | ||
| - | <scene name='10/1037489/Glp1_peptide/2'>Glucagon-like Peptide-1</scene> (GLP-1) is a 30 amino acid hormone secreted into the gut by intestinal epithelial L-cells. GLP-1 is secreted in response to meal intake and is responsible for stimulating insulin production. GLP-1 binding to the GLP-1R located on the membranes of pancreatic cells results in the upregulation of insulin production and secretion which directly results in the lowering of blood glucose levels. <ref name="Holst">PMID:17928588</ref> | + | <scene name='10/1037489/Glp1_peptide/2'>Glucagon-like Peptide-1</scene> (GLP-1) is a 30 amino acid hormone secreted into the gut by intestinal epithelial L-cells. GLP-1 is secreted in response to meal intake and is responsible for stimulating insulin production. GLP-1 binding to the <scene name='10/1037491/Glp-1r/5'>GLP-1R</scene> located on the membranes of pancreatic cells results in the upregulation of insulin production and secretion which directly results in the lowering of blood glucose levels. <ref name="Holst">PMID:17928588</ref> |
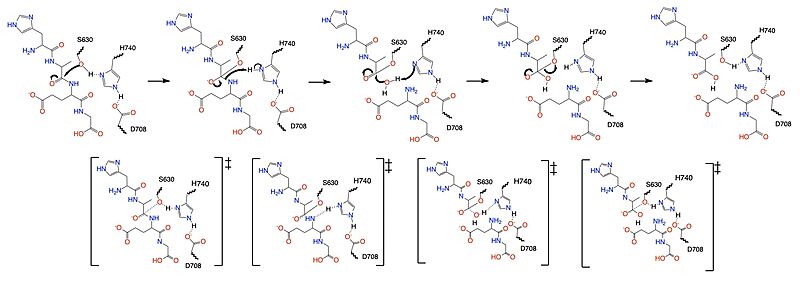
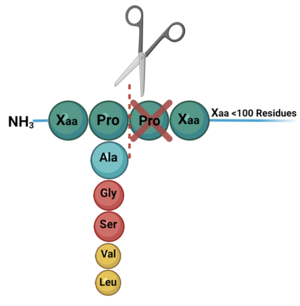
DPP-IV binds and degrades GLP-1 <scene name='10/1037489/Glp1_peptide__scissile_bond/3'>(cleaving at the penultimate position)</scene> resulting in heightened blood glucose levels. Insufficient GLP-1 production or signaling in response to meal intake is clinically associated with T2DM and morbidity. Since GLP-1 is a potent regulator of blood glucose levels, and DPP-IV antagonistically regulates GLP-1, this makes DPP-IV inhibitors an excellent candidate for pharmacological therapeutics for T2DM. <ref name="Gilbert">PMID:32308645</ref> | DPP-IV binds and degrades GLP-1 <scene name='10/1037489/Glp1_peptide__scissile_bond/3'>(cleaving at the penultimate position)</scene> resulting in heightened blood glucose levels. Insufficient GLP-1 production or signaling in response to meal intake is clinically associated with T2DM and morbidity. Since GLP-1 is a potent regulator of blood glucose levels, and DPP-IV antagonistically regulates GLP-1, this makes DPP-IV inhibitors an excellent candidate for pharmacological therapeutics for T2DM. <ref name="Gilbert">PMID:32308645</ref> | ||
Revision as of 16:03, 29 April 2024
Structure and Function of Dipeptidyl Peptidase IV (DPP-IV) in Humans
| |||||||||||
References
- ↑ 1.0 1.1 Ahrén B. DPP-4 Inhibition and the Path to Clinical Proof. Front Endocrinol (Lausanne). 2019 Jun 19;10:376. PMID:31275243 doi:10.3389/fendo.2019.00376
- ↑ Khalse M, Bhargava A. A Review on Cardiovascular Outcome Studies of Dipeptidyl Peptidase-4 Inhibitors. Indian J Endocrinol Metab. 2018 Sep-Oct;22(5):689-695. PMID:30294582 doi:10.4103/ijem.IJEM_104_18
- ↑ Hocher B, Reichetzeder C, Alter ML. Renal and cardiac effects of DPP4 inhibitors--from preclinical development to clinical research. Kidney Blood Press Res. 2012;36(1):65-84. PMID:22947920 doi:10.1159/000339028
- ↑ Zhong J, Rajagopalan S. Dipeptidyl Peptidase-4 Regulation of SDF-1/CXCR4 Axis: Implications for Cardiovascular Disease. Front Immunol. 2015 Sep 25;6:477. PMID:26441982 doi:10.3389/fimmu.2015.00477
- ↑ Sharma A, Ren X, Zhang H, Pandey GN. Effect of depression and suicidal behavior on neuropeptide Y (NPY) and its receptors in the adult human brain: A postmortem study. Prog Neuropsychopharmacol Biol Psychiatry. 2022 Jan 10;112:110428. PMID:34411658 doi:10.1016/j.pnpbp.2021.110428
- ↑ Ntafam CN, Beutler BD, Harris RD. Incarcerated gravid uterus: A rare but potentially devastating obstetric complication. Radiol Case Rep. 2022 Mar 10;17(5):1583-1586. PMID:35309386 doi:10.1016/j.radcr.2022.02.034
- ↑ Chung KM, Cheng JH, Suen CS, Huang CH, Tsai CH, Huang LH, Chen YR, Wang AH, Jiaang WT, Hwang MJ, Chen X. The dimeric transmembrane domain of prolyl dipeptidase DPP-IV contributes to its quaternary structure and enzymatic activities. Protein Sci. 2010 Sep;19(9):1627-38. PMID:20572019 doi:10.1002/pro.443
- ↑ Abbott CA, McCaughan GW, Levy MT, Church WB, Gorrell MD. Binding to human dipeptidyl peptidase IV by adenosine deaminase and antibodies that inhibit ligand binding involves overlapping, discontinuous sites on a predicted beta propeller domain. Eur J Biochem. 1999 Dec;266(3):798-810. PMID:10583373 doi:10.1046/j.1432-1327.1999.00902.x
- ↑ Dobers J, Grams S, Reutter W, Fan H. Roles of cysteines in rat dipeptidyl peptidase IV/CD26 in processing and proteolytic activity. Eur J Biochem. 2000 Aug;267(16):5093-100. PMID:10931192 doi:10.1046/j.1432-1327.2000.01571.x
- ↑ Metzler WJ, Yanchunas J, Weigelt C, Kish K, Klei HE, Xie D, Zhang Y, Corbett M, Tamura JK, He B, Hamann LG, Kirby MS, Marcinkeviciene J. Involvement of DPP-IV catalytic residues in enzyme-saxagliptin complex formation. Protein Sci. 2008 Feb;17(2):240-50. PMID:18227430 doi:17/2/240
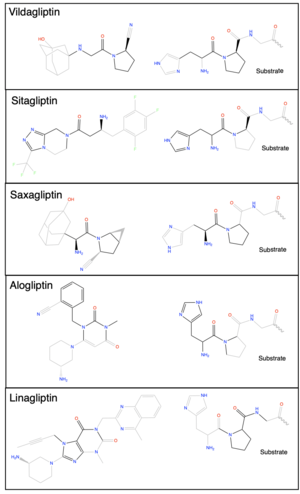
- ↑ 11.0 11.1 Kim BR, Kim HY, Choi I, Kim JB, Jin CH, Han AR. DPP-IV Inhibitory Potentials of Flavonol Glycosides Isolated from the Seeds of Lens culinaris: In Vitro and Molecular Docking Analyses. Molecules. 2018 Aug 10;23(8):1998. PMID:30103438 doi:10.3390/molecules23081998
- ↑ Scott LJ. Sitagliptin: A Review in Type 2 Diabetes. Drugs. 2017 Feb;77(2):209-224. PMID:28078647 doi:10.1007/s40265-016-0686-9
- ↑ Henness S, Keam SJ. Vildagliptin. Drugs. 2006;66(15):1989-2001; discussion 2002-4. PMID:17100408 doi:10.2165/00003495-200666150-00007
- ↑ Garnock-Jones KP. Saxagliptin/Dapagliflozin: A Review in Type 2 Diabetes Mellitus. Drugs. 2017 Mar;77(3):319-330. PMID:28176222 doi:10.1007/s40265-017-0697-1
- ↑ Keating GM. Alogliptin: a review of its use in patients with type 2 diabetes mellitus. Drugs. 2015 May;75(7):777-96. PMID:25855222 doi:10.1007/s40265-015-0385-y
- ↑ Mulvihill EE, Drucker DJ. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr Rev. 2014 Dec;35(6):992-1019. PMID:25216328 doi:10.1210/er.2014-1035
- ↑ Hughes TE, Mone MD, Russell ME, Weldon SC, Villhauer EB. NVP-DPP728 (1-[[[2-[(5-cyanopyridin-2-yl)amino]ethyl]amino]acetyl]-2-cyano-(S)- pyrrolidine), a slow-binding inhibitor of dipeptidyl peptidase IV. Biochemistry. 1999 Sep 7;38(36):11597-603. PMID:10512614 doi:10.1021/bi990852f
- ↑ Banday MZ, Sameer AS, Nissar S. Pathophysiology of diabetes: An overview. Avicenna J Med. 2020 Oct 13;10(4):174-188. PMID:33437689 doi:10.4103/ajm.ajm_53_20
- ↑ Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007 Oct;87(4):1409-39. PMID:17928588 doi:10.1152/physrev.00034.2006
- ↑ Gilbert MP, Pratley RE. GLP-1 Analogs and DPP-4 Inhibitors in Type 2 Diabetes Therapy: Review of Head-to-Head Clinical Trials. Front Endocrinol (Lausanne). 2020 Apr 3;11:178. PMID:32308645 doi:10.3389/fendo.2020.00178
- ↑ Puddu A, Maggi D. Emerging Role of Caveolin-1 in GLP-1 Action. Front Endocrinol (Lausanne). 2021 Apr 14;12:668012. PMID:33935978 doi:10.3389/fendo.2021.668012
Student Contributors
- Karisma Moll
- Merritt Jugo
- Sam Magnabosco