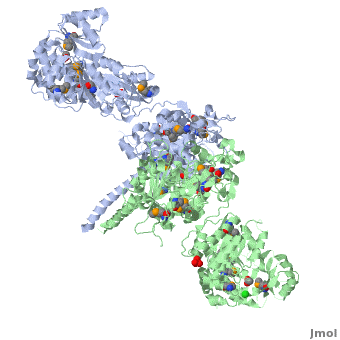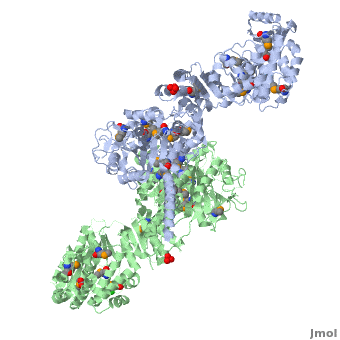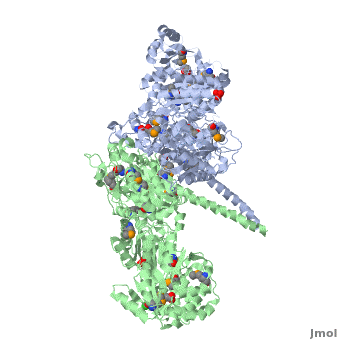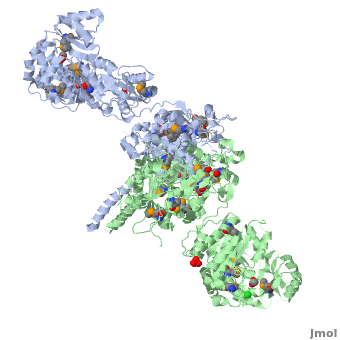
6-deoxyerythronolide B synthase (DEBS)
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
[[Image:DEBS.png|left|500px|thumb|6-deoxyerythronolide B synthase (DEBS)]] | [[Image:DEBS.png|left|500px|thumb|6-deoxyerythronolide B synthase (DEBS)]] | ||
{{Clear}} | {{Clear}} | ||
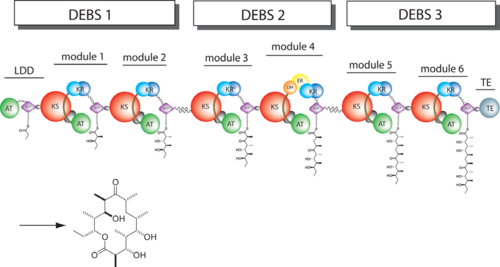
| - | The '''6-deoxyerythronolide B synthase''' (DEBS), which catalyzes the formation of 6-dEB, consists of three large subunits, DEBS1, DEBS2 and DEBS3, each containing two modules and above 300 kD in size. There are 2 domains in the N-terminal loading module, responsible for priming the synthase with a proprionate starter unit, and 26 domains in the six extender modules, Each extender module contains at least three essential domains: a ketosynthase (KS), an acyl transferase (AT) and an acyl carrier protein (ACP). In detail, the AT domain selects the appropriate carbon extender unit and transfers the units from acyl-CoA onto the phosphopantetheine arm of ACP. The KSdomain accepts the polyketide chain from the previous module and catalyzes chain elongation reaction by adding an ACP-bound extender unit through decarboxylative condensation. | + | The '''6-deoxyerythronolide B synthase''' (DEBS), which catalyzes the formation of 6-dEB, consists of three large subunits, DEBS1, DEBS2 and DEBS3, each containing two modules and above 300 kD in size. There are 2 domains in the N-terminal loading module, responsible for priming the synthase with a proprionate starter unit, and 26 domains in the six extender modules, Each extender module contains at least three essential domains: a ketosynthase (KS), an acyl transferase (AT) and an acyl carrier protein (ACP). In detail, the AT domain selects the appropriate carbon extender unit and transfers the units from acyl-CoA onto the phosphopantetheine arm of ACP. The KSdomain accepts the polyketide chain from the previous module and catalyzes chain elongation reaction by adding an ACP-bound extender unit through decarboxylative condensation.<br /> |
| + | |||
| + | '''DEBS1''' is acylated by a diketide at module 2<ref>PMID:8952473</ref>.<br /> | ||
| + | '''DEBS3''' carries out the final two cycles in the synthesis of 6-dEB<ref>PMID:9538011</ref>. | ||
After the extender unit is added, it can be further processed by optional tailoring domains, including ketoreductases (KRs), dehydratases (DHs), and enoyl reductases (ERs), to yield a hydroxyl, enoyl, or methylene group at the beta-position. Finally, the thioesterase (TE) domain that located at the C-terminus of DEBS module 6 promotes the macrocyclization event which releases the final product, 6-dEB. <ref>PMID:17328673</ref> | After the extender unit is added, it can be further processed by optional tailoring domains, including ketoreductases (KRs), dehydratases (DHs), and enoyl reductases (ERs), to yield a hydroxyl, enoyl, or methylene group at the beta-position. Finally, the thioesterase (TE) domain that located at the C-terminus of DEBS module 6 promotes the macrocyclization event which releases the final product, 6-dEB. <ref>PMID:17328673</ref> | ||
Revision as of 09:51, 19 May 2024
| |||||||||||
References
- ↑ Tsukamoto N, Chuck JA, Luo G, Kao CM, Khosla C, Cane DE. 6-deoxyerythronolide B synthase 1 is specifically acylated by a diketide intermediate at the beta-ketoacyl-acyl carrier protein synthase domain of module 2. Biochemistry. 1996 Dec 3;35(48):15244-8. PMID:8952473 doi:10.1021/bi961972f
- ↑ Jacobsen JR, Cane DE, Khosla C. Spontaneous priming of a downstream module in 6-deoxyerythronolide B synthase leads to polyketide biosynthesis. Biochemistry. 1998 Apr 7;37(14):4928-34. PMID:9538011 doi:10.1021/bi9729920
- ↑ Khosla C, Tang Y, Chen AY, Schnarr NA, Cane DE. Structure and mechanism of the 6-deoxyerythronolide B synthase. Annu Rev Biochem. 2007;76:195-221. PMID:17328673 doi:http://dx.doi.org/10.1146/annurev.biochem.76.053105.093515
Additional Resources
For additional information, see: Cancer
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Tsung-Yi Lin, Alexander Berchansky, Lawrence Sheringham Borketey, Joel L. Sussman, Jon Amoroso, David Canner, Jaime Prilusky