Amylase
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
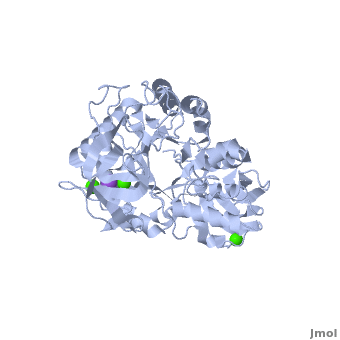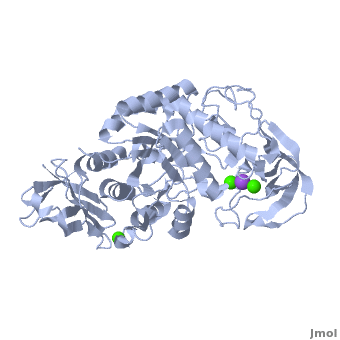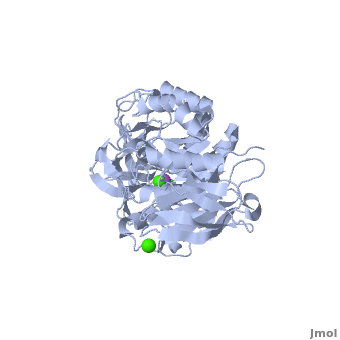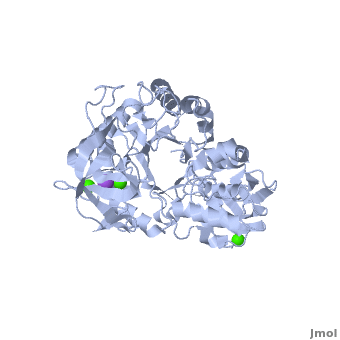
<StructureSection load='' size='350' side='right' scene='Sandbox_182/Alpha-amylase/1' caption='Amylase complex with Ca+2 (green) and Na+ (purple) ions (PDB code [[1hvx]])'> | <StructureSection load='' size='350' side='right' scene='Sandbox_182/Alpha-amylase/1' caption='Amylase complex with Ca+2 (green) and Na+ (purple) ions (PDB code [[1hvx]])'> | ||
=Introduction= | =Introduction= | ||
| - | Discovered and isolated by [http://en.wikipedia.org/wiki/Anselme_Payen Anselme Payen] in 1833, amylase was the first enzyme to be discovered<ref name="book">Yamamoto T.1988. Handbook of Amylases and Related Enzymes: Their Sources, Isolation Methods, Properties and Applications. Osaka Japan: Pergamon Press</ref>. Amylases are hydrolases, acting on α-1,4-glycosidic bonds<ref name="Path">PMID:9541387</ref>. They can be further subdivided into α,β and γ amylases<ref name="book"/>.'''α-Amylase''' (AAM) is an enzyme that acts as a catalyst for the hydrolysis of α-linked polysaccharides into α-anomeric products<ref name="Main">PMID:11226887</ref>. The enzyme can be derived from a variety of sources, each with different characteristics. α-Amylase found within the human body serves as the enzyme active in pancreatic juice and saliva<ref name="Path"/>. α-Amylase is not only essential in human physiology but has a number of important biotechnological functions in various processing industries. '''β/α amylase''' (BAAM) is a precursor protein which is cleaved to form the β-amylase and α-amylase after secretion. '''β amylase''' (BAM) acts at the non-reducing chain ends and liberate only β-maltose<ref>PMID:6168260</ref>. '''γ amylase''' (GAM) acts at the non-reducing chain ends of amylose and amylopectin and liberates glucose. '''Pullulanase''' hydrolyses the α-1,6 glucoside linkage in starch, amylopectin, pullulan and related oligosaccharides<ref>PMID:22991654</ref>.<br /> | + | Discovered and isolated by [http://en.wikipedia.org/wiki/Anselme_Payen Anselme Payen] in 1833, '''amylase''' was the first enzyme to be discovered<ref name="book">Yamamoto T.1988. Handbook of Amylases and Related Enzymes: Their Sources, Isolation Methods, Properties and Applications. Osaka Japan: Pergamon Press</ref>. Amylases are hydrolases, acting on α-1,4-glycosidic bonds<ref name="Path">PMID:9541387</ref>. They can be further subdivided into α,β and γ amylases<ref name="book"/>.'''α-Amylase''' (AAM) is an enzyme that acts as a catalyst for the hydrolysis of α-linked polysaccharides into α-anomeric products<ref name="Main">PMID:11226887</ref>. The enzyme can be derived from a variety of sources, each with different characteristics. α-Amylase found within the human body serves as the enzyme active in pancreatic juice and saliva<ref name="Path"/>. α-Amylase is not only essential in human physiology but has a number of important biotechnological functions in various processing industries. '''β/α amylase''' (BAAM) is a precursor protein which is cleaved to form the β-amylase and α-amylase after secretion. '''β amylase''' (BAM) acts at the non-reducing chain ends and liberate only β-maltose<ref>PMID:6168260</ref>. '''γ amylase''' (GAM) acts at the non-reducing chain ends of amylose and amylopectin and liberates glucose. '''Pullulanase''' hydrolyses the α-1,6 glucoside linkage in starch, amylopectin, pullulan and related oligosaccharides<ref>PMID:22991654</ref>.<br /> |
For α-amylase see [[Raghad zoubi]]<br /> | For α-amylase see [[Raghad zoubi]]<br /> | ||
See also [[Amylase (Hebrew)]]. | See also [[Amylase (Hebrew)]]. | ||
Revision as of 09:13, 26 May 2024
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Yamamoto T.1988. Handbook of Amylases and Related Enzymes: Their Sources, Isolation Methods, Properties and Applications. Osaka Japan: Pergamon Press
- ↑ 2.0 2.1 Aghajari N, Feller G, Gerday C, Haser R. Crystal structures of the psychrophilic alpha-amylase from Alteromonas haloplanctis in its native form and complexed with an inhibitor. Protein Sci. 1998 Mar;7(3):564-72. PMID:9541387
- ↑ 3.0 3.1 3.2 Suvd D, Fujimoto Z, Takase K, Matsumura M, Mizuno H. Crystal structure of Bacillus stearothermophilus alpha-amylase: possible factors determining the thermostability. J Biochem. 2001 Mar;129(3):461-8. PMID:11226887
- ↑ French D. Amylases: enzymatic mechanisms. Basic Life Sci. 1981;18:151-82. PMID:6168260
- ↑ Hii SL, Tan JS, Ling TC, Ariff AB. Pullulanase: role in starch hydrolysis and potential industrial applications. Enzyme Res. 2012;2012:921362. doi: 10.1155/2012/921362. Epub 2012 Sep 6. PMID:22991654 doi:http://dx.doi.org/10.1155/2012/921362
- ↑ 6.0 6.1 6.2 Aghajari N, Feller G, Gerday C, Haser R. Structural basis of alpha-amylase activation by chloride. Protein Sci. 2002 Jun;11(6):1435-41. PMID:12021442
- ↑ Maurus R, Begum A, Williams LK, Fredriksen JR, Zhang R, Withers SG, Brayer GD. Alternative catalytic anions differentially modulate human alpha-amylase activity and specificity(,). Biochemistry. 2008 Mar 18;47(11):3332-44. Epub 2008 Feb 20. PMID:18284212 doi:10.1021/bi701652t
- ↑ 8.0 8.1 Maurus R, Begum A, Williams LK, Fredriksen JR, Zhang R, Withers SG, Brayer GD. Alternative catalytic anions differentially modulate human alpha-amylase activity and specificity(,). Biochemistry. 2008 Mar 18;47(11):3332-44. Epub 2008 Feb 20. PMID:18284212 doi:10.1021/bi701652t
- ↑ 9.0 9.1 9.2 9.3 Kuriki T, Imanaka T. The concept of the alpha-amylase family: structural similarity and common catalytic mechanism. J Biosci Bioeng. 1999;87(5):557-65. PMID:16232518
- ↑ 10.0 10.1 PPMID: 17713601
- ↑ Franco OL, Rigden DJ, Melo FR, Grossi-De-Sa MF. Plant alpha-amylase inhibitors and their interaction with insect alpha-amylases. Eur J Biochem. 2002 Jan;269(2):397-412. PMID:11856298
- ↑ Yang RW, Shao ZX, Chen YY, Yin Z, Wang WJ. Lipase and pancreatic amylase activities in diagnosis of acute pancreatitis in patients with hyperamylasemia. Hepatobiliary Pancreat Dis Int. 2005 Nov;4(4):600-3. PMID:16286272
- ↑ Agirre J, Moroz O, Meier S, Brask J, Munch A, Hoff T, Andersen C, Wilson KS, Davies GJ. The structure of the AliC GH13 alpha-amylase from Alicyclobacillus sp. reveals the accommodation of starch branching points in the alpha-amylase family. Acta Crystallogr D Struct Biol. 2019 Jan 1;75(Pt 1):1-7. doi:, 10.1107/S2059798318014900. Epub 2019 Jan 4. PMID:30644839 doi:http://dx.doi.org/10.1107/S2059798318014900
- ↑ Machius M, Declerck N, Huber R, Wiegand G. Activation of Bacillus licheniformis alpha-amylase through a disorder-->order transition of the substrate-binding site mediated by a calcium-sodium-calcium metal triad. Structure. 1998 Mar 15;6(3):281-92. PMID:9551551
- ↑ Brzozowski AM, Lawson DM, Turkenburg JP, Bisgaard-Frantzen H, Svendsen A, Borchert TV, Dauter Z, Wilson KS, Davies GJ. Structural analysis of a chimeric bacterial alpha-amylase. High-resolution analysis of native and ligand complexes. Biochemistry. 2000 Aug 8;39(31):9099-107. PMID:10924103
Proteopedia Page Contributors and Editors (what is this?)
Shane Riley, Michal Harel, Joel L. Sussman, Randi Woodbeck, Jaime Prilusky, Alexander Berchansky, Ann Taylor, Andrea Gorrell, David Canner