User:Maria Júlia Pasian Ferreira/Sandbox 2
From Proteopedia
| Line 8: | Line 8: | ||
== Class 1 == | == Class 1 == | ||
| - | <StructureSection load='Crystal structure of PhaC1 from Ralstonia eutropha.pdb' size='350' side='right' caption='Crystal structure of PhaC1 from Ralstonia eutropha (PDB entry [[5HZ2]])' scene='10/1050300/Phac_class_i/1'>The class 1 phaCs synthetize preferentially short chain PHAs, with 3 to 5 carbon monomers, and are composed by a | + | <StructureSection load='Crystal structure of PhaC1 from Ralstonia eutropha.pdb' size='350' side='right' caption='Crystal structure of PhaC1 from Ralstonia eutropha (PDB entry [[5HZ2]])' scene='10/1050300/Phac_class_i/1'>The class 1 phaCs synthetize preferentially short chain PHAs, with 3 to 5 carbon monomers, and are composed by a single enzymatic unit, with molecular mass ranging from 63 to 73 kDA. (Chek et al., 2018; Neoh et al., 2022). |
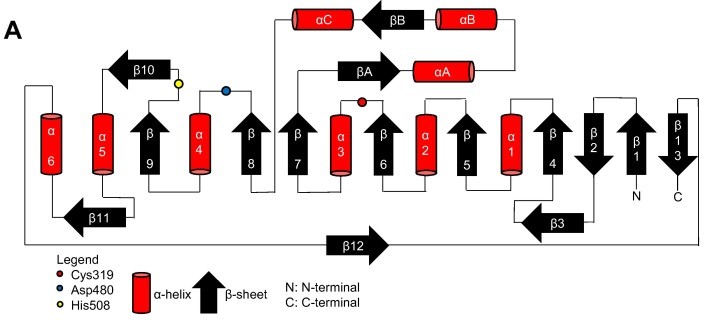
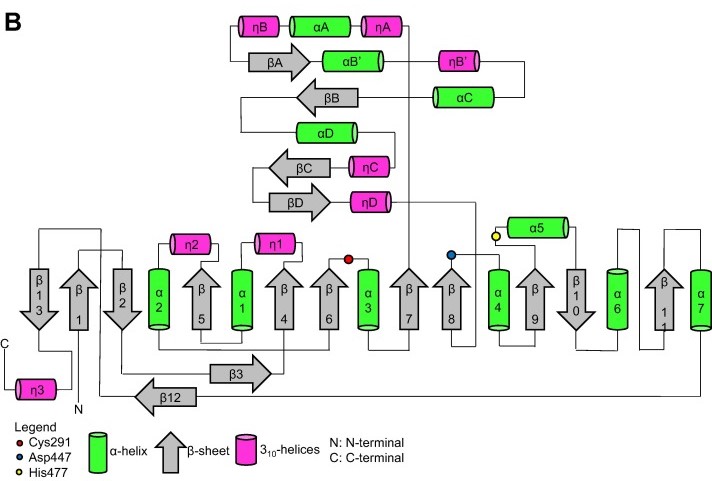
| - | Thanks to the X-ray cristallography data from PhaC<sub>cn</sub>-CAT and PhaC<sub>cs</sub>-CAT, it was possible to categorize the class 1 phaCs in based on their molecular organization, made of two domains: The N-terminal domain and the catalytic C-terminal domain. The C-terminal domain carries the catalytic site, formed by the aminoacid triad (Cys-Asp-His) located deep within the hydrophobic cavity, that in the closed conformation is partially covered by the Cap subdomain.(Chek et al., 2018; Neoh et al., 2022). | + | Thanks to the X-ray cristallography data from PhaC<sub>cn</sub>-CAT and PhaC<sub>cs</sub>-CAT, it was possible to categorize the class 1 phaCs in based on their molecular organization, made of two domains: The N-terminal domain and the <scene name='10/1050300/Phac_i_catalytic_c_domain/1'>catalytic C-terminal domain</scene>. The C-terminal domain carries the <scene name='10/1050300/Phac_i_catalytic_site/2'>catalytic site, formed by the aminoacid triad (Cys-Asp-His)</scene> located deep within the <scene name='10/1050300/Phac_i_polar/1'>hydrophobic cavity</scene>, that in the closed conformation is <scene name='10/1050300/Phac_i_cap_subdomain/1'>partially covered by the Cap subdomain</scene>.(Chek et al., 2018; Neoh et al., 2022). |
=== N-terminal domain === | === N-terminal domain === | ||
| Line 16: | Line 16: | ||
=== C-terminal domain === | === C-terminal domain === | ||
| - | Contrary to the flexible N-terminal domain, the C-terminal domain is relatively stable, making its crytalization process easier. Because of this, it was possible to obtain the C-terminal domain structure from PhaC<sub>cn</sub>-CAT and PhaC<sub>cs</sub>-CAT through X-ray cristallography, with resolution of 1.8 Å and 1.48 Å, respectively. The C-terminal domain has the catalytic site, with the aminoacid triad (Cys-Asp-His), the substrate entrance and the product egress tunnel. | + | Contrary to the flexible N-terminal domain, the <scene name='10/1050300/Phac_i_catalytic_c_domain/2'>C-terminal domain</scene> is relatively stable, making its crytalization process easier. Because of this, it was possible to obtain the C-terminal domain structure from PhaC<sub>cn</sub>-CAT and PhaC<sub>cs</sub>-CAT through X-ray cristallography, with resolution of 1.8 Å and 1.48 Å, respectively. The C-terminal domain has <scene name='10/1050300/Phac_i_catalytic_site/2'>the catalytic site, with the aminoacid triad (Cys-Asp-His)</scene>, the substrate entrance and the product egress tunnel. |
| - | The overall form of a phaC protein is that of a typical protein from the α/β-hydrolase-fold, with the C-terminal domain made of an α/β-hydrolase core subdomain and a Cap subdomain, corresponding to the Thr347-Pro471 residues in PhaC<sub>cn</sub>, and Thr319-Pro438 residues in PhaC<sub>cs</sub>. It is in the α/β-hydrolase subdomain that the entrance tunnel, the catalytic site and the product egress tunnel are located. This region seems to be preserved in phaCs. | + | The overall form of a phaC protein is that of a typical protein from the α/β-hydrolase-fold, with the C-terminal domain made of an <scene name='10/1050300/Phac_i_catalytic_hydrolase_sub/1'>α/β-hydrolase core subdomain</scene> and a <scene name='10/1050300/Phac_i_catalytic_cap_sub/1'>Cap subdomain</scene>, corresponding to the Thr347-Pro471 residues in PhaC<sub>cn</sub>, and Thr319-Pro438 residues in PhaC<sub>cs</sub>. It is in the α/β-hydrolase subdomain that the entrance tunnel, the catalytic site and the product egress tunnel are located. This region seems to be preserved in phaCs. |
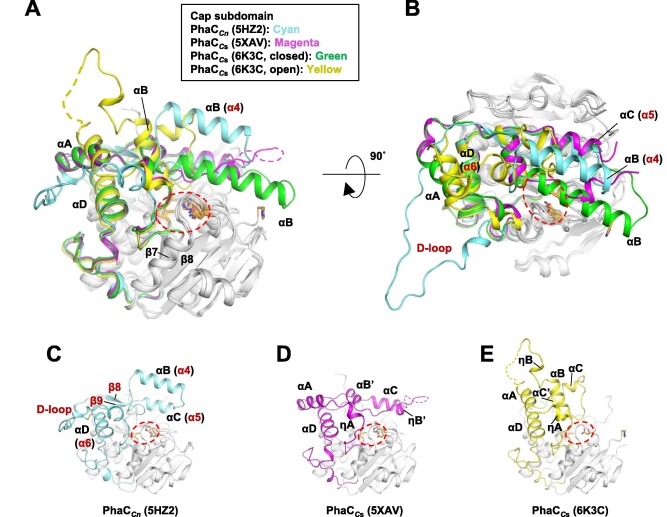
| - | Regarding the Cap subdomain, the LID region is extremely dynamic and flexible, having an open or closed conformation based on structural changes. Because of this, the Cap subdomain, specially the LID region, is not as conserverd in the phaCs as the α/β-hydrolase subdomain. The Cap subdomain is located after the β7 sheet, and connects with the β8 sheet from the α/β-hydrolase core. In PhaC<sub>cn</sub>, the Cap subdomain is formed by three α-helixes (α4, α5 and α6) and two β-sheets (β8 and β9). Meanwhile, PhaC<sub>cs</sub> has six α-helixes (αA, αB, αC, αD, ηA and ηB'). | + | Regarding the <scene name='10/1050300/Phac_i_catalytic_cap_sub/1'>Cap subdomain</scene>, the LID region is extremely dynamic and flexible, having an open or closed conformation based on structural changes. Because of this, the Cap subdomain, specially the LID region, is not as conserverd in the phaCs as the α/β-hydrolase subdomain. The Cap subdomain is located after the β7 sheet, and connects with the β8 sheet from the α/β-hydrolase core. In PhaC<sub>cn</sub>, the Cap subdomain is formed by three α-helixes (α4, α5 and α6) and two β-sheets (β8 and β9). Meanwhile, PhaC<sub>cs</sub> has six α-helixes (αA, αB, αC, αD, ηA and ηB'). |
The cristallography of PhaC<sub>cs</sub>-CAT bound to its substrate revealed the complex assymetric dimer structure of this enzyme. The phaC dimer form can be induced by the presence the substrate. Due to the dynamic and flexible properties, specially of the LID region, the Cap subdomain is paramount in the phaC dimer formation and regulation of substrate entry and product release, since it determines the protomer's movements, regulating the change between the closed form - homodimer- and the open form - heterodimer. (Neoh et al., 2022). | The cristallography of PhaC<sub>cs</sub>-CAT bound to its substrate revealed the complex assymetric dimer structure of this enzyme. The phaC dimer form can be induced by the presence the substrate. Due to the dynamic and flexible properties, specially of the LID region, the Cap subdomain is paramount in the phaC dimer formation and regulation of substrate entry and product release, since it determines the protomer's movements, regulating the change between the closed form - homodimer- and the open form - heterodimer. (Neoh et al., 2022). | ||
Current revision
Contents |
Introduction
Poli 3-hydroxyalcanoates (PHA) are the main components of the cytoplasmatic inclusions, called PHA granules. They serve as carbon and energy reserves in many species of procariotes. PHA polymers can be made from different sized monomers, ranging from 4 to 14 carbon atoms. They are typically synthetized when there is an unbalanced distribution of nutrients in the medium, mainly an abundance of carbon sources coupled with the lack of another essential component. Among the existing PHAs, the most common are the poli-3-hydroxybutirate (PHB) polymers. (Barbosa, Gomez, Torres, 2018, p. 52). PHBs are specially relevant due to their properties that resemble conventional plastics, such as polypropylene. Since they are biopolymers made from renewable, biodegrabable and compatible source materials, PHBs present themselves as an industrial alternative to petrol-based plastics. (Byrom, 1987; Madison and Huisman 1999). The polymerization of PHA monomers is performed by the PHA sintase/polymerase (phaC) enzyme. At first, two acetyl-CoA molecules are condensed into one acetoacetyl-CoA by a β-ketoacyl-CoA thiolase, and then reduced to (R)-3-hydroxybutyryl-CoA by the acetoacetyl-CoA dehydrogenase. Finally, phaC polymerizes the (R)-3-hydroxybutyryl-CoA molecules into the PHA polymer. (Reddy et al., 2003).
Currently, there are four known classes of phaC, that are distinguished by their primary structure, substrate specificity and subunits composition (Rhem, 2008; Neoh et al., 2022). Despite vast diversity, only the catalytic domain of the PhaCcn-CAT from Ralstonia eutropha H16 and the USM2 PhaCcs-CAT from the Chromobacterium sp., both being class 1 phaCs. (Neoh et al., 2022).
Class 1
| |||||||||||
|
Class 2
Among the 4 classes of phaC, are the only that synthetize preferentially medium chain PHA, with 6 to 14 carbon monomers. Just like the class 1, they are also formed by a single enzymatic subunit, however, its molecular mass is, in general, higher than class 1 phaC, with approximately 62 kDa. It is suggested that the class 2 molecules also have two domains. (Chek et al., 2018).
Class 3
produce preferentially short chain PHAs, with 3 to 5 carbon monomers. However, differently from classes 1 and 2, , and has a smaller molecular mass ranging from 40 to 53 kDa. (Chek et al., 2018; Jia et al., 2016).
Class 4
Just like class 3, and , and has a smaller molecular mass than the other classes. The class 4 phaC also produce preferentially short chain PHAs, with 3 to 5 carbon monomers.