Arabidospsis thaliana PrRs (pinoresinol/lariciresinol reductases)
From Proteopedia
| Line 9: | Line 9: | ||
Pinoresinol/Lariciresinol Reductases (PLR) are NADPH-dependent reductases that constitute a family of enzymes involved in the lignans biosynthetic pathway (Xiao et al., 2021). Lignans are a group of nearly 2000 (Xiao et al., 2021) secondary metabolites, formed by dimers of phenylpropanoids, that can be present in all organs of some non-vascular plants, some Tracheophytes, such as Pteridophytes, and Gymnosperms (Markulin et al., 2019). Although their biological role is not yet totally clarified, they are associated mainly with plants defense mechanisms, especially towards herbivores and pathogenic microorganisms (Markulin et al., 2019), providing antibacterial, antifungal and antifeedant effects (Markulin et al., 2019), besides many other impacts. | Pinoresinol/Lariciresinol Reductases (PLR) are NADPH-dependent reductases that constitute a family of enzymes involved in the lignans biosynthetic pathway (Xiao et al., 2021). Lignans are a group of nearly 2000 (Xiao et al., 2021) secondary metabolites, formed by dimers of phenylpropanoids, that can be present in all organs of some non-vascular plants, some Tracheophytes, such as Pteridophytes, and Gymnosperms (Markulin et al., 2019). Although their biological role is not yet totally clarified, they are associated mainly with plants defense mechanisms, especially towards herbivores and pathogenic microorganisms (Markulin et al., 2019), providing antibacterial, antifungal and antifeedant effects (Markulin et al., 2019), besides many other impacts. | ||
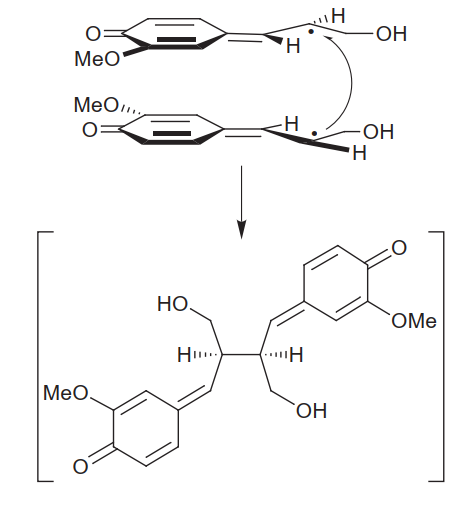
| - | Lignans are synthesized by the dimerization of 2 phenylpropanoids: [[Image:Síntese de lignanas.png | + | Lignans are synthesized by the dimerization of 2 phenylpropanoids: [[Image:Síntese de lignanas.png]] |
The presence or absence of the 8’,8’ bond between phenylpropanoid monomers differentiate lignans from neolignans, however overall both are associated with many biological activities in humans, due to their structural diversity (Xiao et al., 2021), including antiinflammatory, antioxidant, neuroprotective, antiviral, insecticidal and antitumor proliferation effects (Wang et al., 2022). Given the possibility of positively impacting human health, and their large diversity, lignans are thoroughly explored by the pharmaceutical industry (Markulin et al., 2019). | The presence or absence of the 8’,8’ bond between phenylpropanoid monomers differentiate lignans from neolignans, however overall both are associated with many biological activities in humans, due to their structural diversity (Xiao et al., 2021), including antiinflammatory, antioxidant, neuroprotective, antiviral, insecticidal and antitumor proliferation effects (Wang et al., 2022). Given the possibility of positively impacting human health, and their large diversity, lignans are thoroughly explored by the pharmaceutical industry (Markulin et al., 2019). | ||
Revision as of 15:41, 2 June 2024
Página sobre a PrR1 e PrR2 de Arabidopsis feita por alunos da Biologia da USP São Paulo.
A. thaliana PrRs
| |||||||||||
4. AtPrR expression
Understanding the pattern of expression of the PLR family is key in the study of lignans, considering that the diversity of these secondary metabolites is due to the action of these enzymes, but also to their expression pattern (Xiao et al., 2021; Markulin et al., 2019), which dictates where in the plants and when a certain lignan will be produced.
The pattern of expression of the PLRs varies from plant to plant and it can be altered by different stresses and hormones (Markunil et al., 2019). In the case of Arabidopsis thaliana, both PrR1 and PrR2 are expressed in root, but only PrR1 is also expressed in stem. Zhao and collaborators (2014) have shown by co-expression analysis that PrR1 might be related to the deposition of secondary cell wall, as it clustered with genes related to: lignification, hemicellulose biosynthesis, cellulose synthesis, etc. This possibility was reinforced by transactivation assays in which the trans-activators factors MYB46 and SND1, both related to the deposition of secondary cell wall, interacted with the PrR1 promoter. On other hand, PrR2 clustered with a different set of genes, been close to genes not related to secondary cell wall deposition, as well to genes related to lignification and to the formation of the casparian strip.
What might look contradictory is the fact that PrR1 and PrR2 are coexpressed with lignin related genes and that pinoresinol, the first substrate of the PLR enzymes, is synthetized by the dimerization of two coniferyl alcohols, onde of the monomers that constitute the lignin polymer. We could imagine that the production of lignans would compete for substrates with the biosynthesis of lignin, and therefore the coexpression mentioned would be questionable. Zhao and collaborators (2014) have shown that mutants of A. thaliana without the function of the PrR1 enzyme show lower content of lignin, meaning that, somehow the production of lignans is important for lignification and related to lignin polymerization.
5. References
MARKULIN, L. et al. Pinoresinol–lariciresinol reductases, key to the lignan synthesis in plants. Planta, v. 249, n. 6, p. 1695–1714, 20 mar. 2019. PMID: 30895445. DOI: https://doi.org/10.1007/s00425-019-03137-y.
TOMOYUKI NAKATSUBO et al. Characterization of Arabidopsis thaliana Pinoresinol Reductase, a New Type of Enzyme Involved in Lignan Biosynthesis. Journal of Biological Chemistry, v. 283, n. 23, p. 15550–15557, 1 jun. 2008. PMID: 18347017. DOI: https://doi.org/10.1074%2Fjbc.M801131200.
XIAO, Y. et al. Structure-based engineering of substrate specificity for pinoresinol-lariciresinol reductases. Nature communications, v. 12, n. 1, 14 maio 2021. PMID: 33990581. DOI: https://doi.org/10.1038/s41467-021-23095-y.
ZHAO, Q. et al. Pinoresinol reductase 1 impacts lignin distribution during secondary cell wall biosynthesis in Arabidopsis. Phytochemistry, v. 112, p. 170–178, 1 abr. 2015. PMID: 25107662. DOI: https://doi.org/10.1016/j.phytochem.2014.07.008.
Proteopedia Page Contributors and Editors (what is this?)
Gabriel Fontanella Pileggi, Maisa Ganz Sanchez Sennes, Melissa Siolin Martins, Michal Harel, Angel Herraez