Laccase
From Proteopedia
(Difference between revisions)
| Line 2: | Line 2: | ||
== Function == | == Function == | ||
| - | '''Laccase''' (Lac) or '''multicopper oxidase''' is a multi-copper protein which uses molecular oxygen to oxidize various aromatic and non-aromatic compounds by a radical-catalyzed reaction mechanism<ref>PMID:15036303</ref>. The multi-copper oxidases constitute a family of enzymes whose principal members are laccase (benzenediol oxygen oxidoreductase, EC 1.10.3.2), ascorbate oxidase (L-ascorbate oxygen oxidoreductase, EC 1.10.3.3) and ceruloplasmin (Fe(II) oxygen oxidoreductase, EC 1.16.3.1). | + | '''Laccase''' (Lac) or '''multicopper oxidase''' is a multi-copper protein which uses molecular oxygen to oxidize various aromatic and non-aromatic compounds by a radical-catalyzed reaction mechanism<ref>PMID:15036303</ref>. The multi-copper oxidases constitute a family of enzymes whose principal members are laccase (benzenediol oxygen oxidoreductase, EC 1.10.3.2), ascorbate oxidase (L-ascorbate oxygen oxidoreductase, EC 1.10.3.3) and ceruloplasmin (Fe(II) oxygen oxidoreductase, EC 1.16.3.1). |
| - | + | ||
* '''Laccase 2''' (Lac2) acts in lignin degradation and in detoxification of lignin products. Typically, laccases show a three cupredoxin-domain folding<ref>PMID:25586560</ref>. | * '''Laccase 2''' (Lac2) acts in lignin degradation and in detoxification of lignin products. Typically, laccases show a three cupredoxin-domain folding<ref>PMID:25586560</ref>. | ||
*'''Two-domain laccase''' or small lactase have unusual resistance to inhibitors<ref>PMID:25778839</ref>. | *'''Two-domain laccase''' or small lactase have unusual resistance to inhibitors<ref>PMID:25778839</ref>. | ||
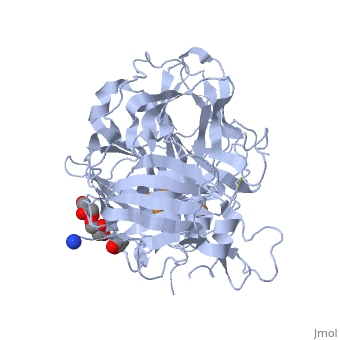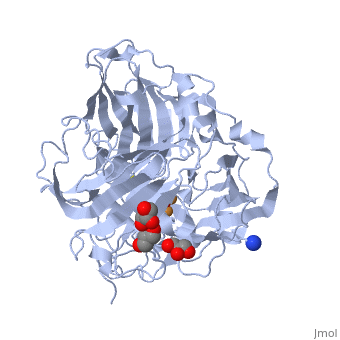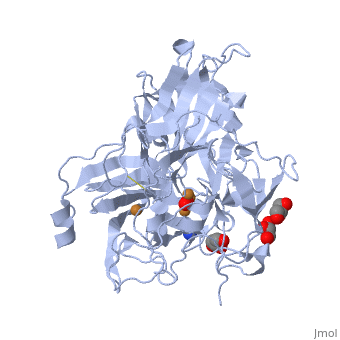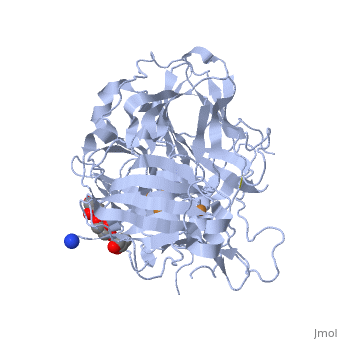
| - | *'''CotA laccase''' belongs to the multi-copper oxidase family. | + | *'''CotA laccase''' belongs to the multi-copper oxidase family. Similar to the other laccases the three dimensional structure of CotA [[1w6l]] comprises three cupredoxin domains and four copper ions organised in <scene name='CotA_laccase/Copper_centers/5'>Two copper centers</scene>: |
| + | a <scene name='40/404916/Cv/3'>mononuclear blue type 1 copper center</scene> and <scene name='40/404916/Cv/2'>a trinuclear center</scene>.<ref>PMID:11514528</ref><ref>PMID:16234932</ref> | ||
For laccase with nitrotyrosine modification see [[Nitrotyrosine]]. | For laccase with nitrotyrosine modification see [[Nitrotyrosine]]. | ||
Current revision
| |||||||||||
References
- ↑ Claus H. Laccases: structure, reactions, distribution. Micron. 2004;35(1-2):93-6. doi: 10.1016/j.micron.2003.10.029. PMID:15036303 doi:http://dx.doi.org/10.1016/j.micron.2003.10.029
- ↑ Pardo I, Camarero S. Laccase engineering by rational and evolutionary design. Cell Mol Life Sci. 2015 Mar;72(5):897-910. doi: 10.1007/s00018-014-1824-8. Epub, 2015 Jan 14. PMID:25586560 doi:http://dx.doi.org/10.1007/s00018-014-1824-8
- ↑ Trubitsina LI, Tishchenko SV, Gabdulkhakov AG, Lisov AV, Zakharova MV, Leontievsky AA. Structural and functional characterization of two-domain laccase from Streptomyces viridochromogenes. Biochimie. 2015 May;112:151-9. doi: 10.1016/j.biochi.2015.03.005. Epub 2015 Mar, 13. PMID:25778839 doi:http://dx.doi.org/10.1016/j.biochi.2015.03.005
- ↑ Hullo MF, Moszer I, Danchin A, Martin-Verstraete I. CotA of Bacillus subtilis is a copper-dependent laccase. J Bacteriol. 2001 Sep;183(18):5426-30. PMID:11514528
- ↑ Bento I, Martins LO, Gato Lopes G, Armenia Carrondo M, Lindley PF. Dioxygen reduction by multi-copper oxidases; a structural perspective. Dalton Trans. 2005 Nov 7;(21):3507-13. Epub 2005 Sep 27. PMID:16234932 doi:10.1039/b504806k
- ↑ Bankole PO, Adekunle AA, Govindwar SP. Demethylation and desulfonation of textile industry dye, Thiazole Yellow G by Aspergillus niger LAG. Biotechnol Rep (Amst). 2019 Mar 28;23:e00327. doi: 10.1016/j.btre.2019.e00327., eCollection 2019 Sep. PMID:30997348 doi:http://dx.doi.org/10.1016/j.btre.2019.e00327
- ↑ Shraddha, Shekher R, Sehgal S, Kamthania M, Kumar A. Laccase: microbial sources, production, purification, and potential biotechnological applications. Enzyme Res. 2011;2011:217861. doi: 10.4061/2011/217861. Epub 2011 Jun 21. PMID:21755038 doi:http://dx.doi.org/10.4061/2011/217861
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Isabel Bento, Alexander Berchansky, David Canner, Jaime Prilusky