We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Monooxygenase
From Proteopedia
(Difference between revisions)
| Line 4: | Line 4: | ||
'''Monooxygenases''' (MO) catalyzes the incorporation of a hydroxyl group into a variety of substrates. MO catalyzes the reduction of O<sub>2</sub> to H<sub>2</sub>O while oxidating NADPH. | '''Monooxygenases''' (MO) catalyzes the incorporation of a hydroxyl group into a variety of substrates. MO catalyzes the reduction of O<sub>2</sub> to H<sub>2</sub>O while oxidating NADPH. | ||
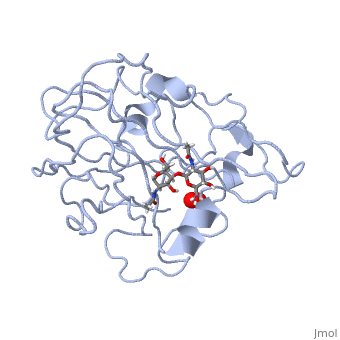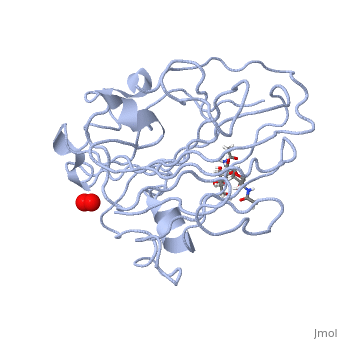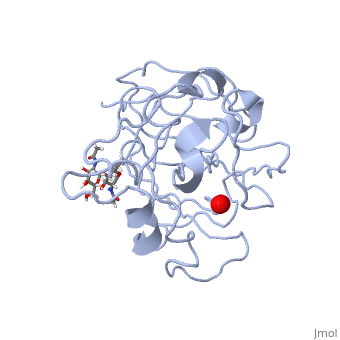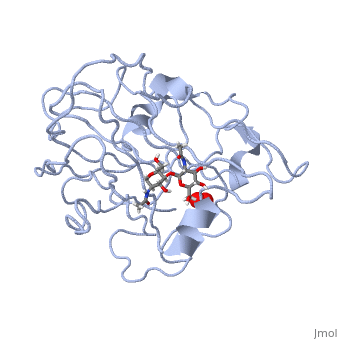
| + | *'''ActVA-Orf6 monooxygenase''' catalyses the oxidation of an aromatic intermediate of the actinorhodin pathway <ref>PMID:12514125</ref>. | ||
=== Peptidylglycine α-Hydroxylating Monooxygenase (PHM)-coordination of peroxide to Cu<sub>M</sub> center. Structural and computational study <ref >doi 10.1007/s00775-012-0967-z</ref>=== | === Peptidylglycine α-Hydroxylating Monooxygenase (PHM)-coordination of peroxide to Cu<sub>M</sub> center. Structural and computational study <ref >doi 10.1007/s00775-012-0967-z</ref>=== | ||
Revision as of 09:18, 15 July 2024
| |||||||||||
References
- ↑ Olsen DS, Savner EM, Mathew A, Zhang F, Krishnamoorthy T, Phan L, Hinnebusch AG. Domains of eIF1A that mediate binding to eIF2, eIF3 and eIF5B and promote ternary complex recruitment in vivo. EMBO J. 2003 Jan 15;22(2):193-204. PMID:12514125 doi:10.1093/emboj/cdg030
- ↑ Rudzka K, Moreno DM, Eipper B, Mains R, Estrin DA, Amzel LM. Coordination of peroxide to the Cu(M) center of peptidylglycine alpha-hydroxylating monooxygenase (PHM): structural and computational study. J Biol Inorg Chem. 2012 Dec 18. PMID:23247335 doi:10.1007/s00775-012-0967-z
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Joel L. Sussman, Alexander Berchansky, Jaime Prilusky