We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Monooxygenase
From Proteopedia
(Difference between revisions)
| Line 4: | Line 4: | ||
'''Monooxygenases''' (MO) catalyzes the incorporation of a hydroxyl group into a variety of substrates. MO catalyzes the reduction of O<sub>2</sub> to H<sub>2</sub>O while oxidating NADPH. | '''Monooxygenases''' (MO) catalyzes the incorporation of a hydroxyl group into a variety of substrates. MO catalyzes the reduction of O<sub>2</sub> to H<sub>2</sub>O while oxidating NADPH. | ||
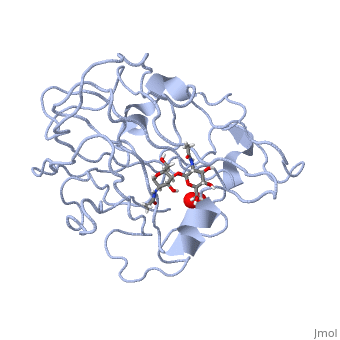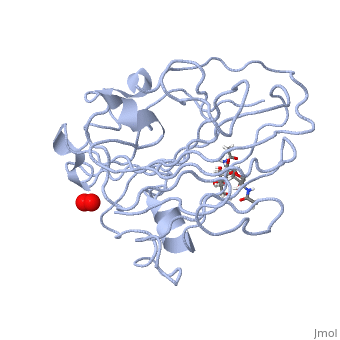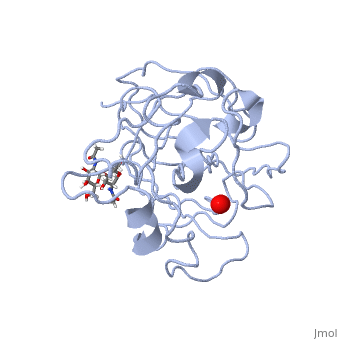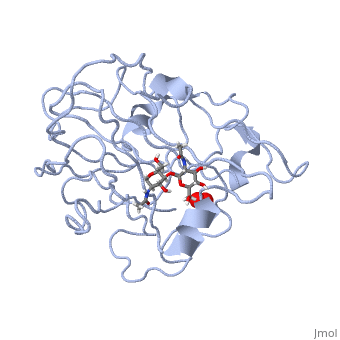
| - | *'''ActVA-Orf6 monooxygenase''' catalyses the oxidation of an aromatic intermediate of the actinorhodin pathway <ref>PMID: | + | *'''ActVA-Orf6 monooxygenase''' catalyses the oxidation of an aromatic intermediate of the actinorhodin pathway <ref>PMID:12514126</ref>. |
=== Peptidylglycine α-Hydroxylating Monooxygenase (PHM)-coordination of peroxide to Cu<sub>M</sub> center. Structural and computational study <ref >doi 10.1007/s00775-012-0967-z</ref>=== | === Peptidylglycine α-Hydroxylating Monooxygenase (PHM)-coordination of peroxide to Cu<sub>M</sub> center. Structural and computational study <ref >doi 10.1007/s00775-012-0967-z</ref>=== | ||
Revision as of 09:18, 15 July 2024
| |||||||||||
References
- ↑ Sciara G, Kendrew SG, Miele AE, Marsh NG, Federici L, Malatesta F, Schimperna G, Savino C, Vallone B. The structure of ActVA-Orf6, a novel type of monooxygenase involved in actinorhodin biosynthesis. EMBO J. 2003 Jan 15;22(2):205-15. PMID:12514126 doi:http://dx.doi.org/10.1093/emboj/cdg031
- ↑ Rudzka K, Moreno DM, Eipper B, Mains R, Estrin DA, Amzel LM. Coordination of peroxide to the Cu(M) center of peptidylglycine alpha-hydroxylating monooxygenase (PHM): structural and computational study. J Biol Inorg Chem. 2012 Dec 18. PMID:23247335 doi:10.1007/s00775-012-0967-z
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Joel L. Sussman, Alexander Berchansky, Jaime Prilusky