Electrostatic potential maps
From Proteopedia
(Difference between revisions)
(→Gallery) |
(→See Also) |
||
| Line 22: | Line 22: | ||
Click on the image to enlarge. | Click on the image to enlarge. | ||
|} | |} | ||
| + | |||
| + | ==Methods== | ||
| + | |||
| + | ===PyMOL=== | ||
| + | # Enter command "fetch 1pgb". | ||
| + | # Menu: All, Action, remove waters. | ||
| + | # Menu: 1pgb, Action, generate, vacuum electrostatics, protein contact potential (local). | ||
==See Also== | ==See Also== | ||
Revision as of 18:25, 25 August 2024
It is revealing to visualize the distribution of electrostatic charges, electrostatic potential, on molecular surfaces. Most protein-protein and protein-ligand interactions are largely electrostatic in nature, via hydrogen bonds and ionic interactions. Their strengths are modulated by the nature of the solvent: pure water or high ionic strength aqueous solution.
Contents |
Gallery
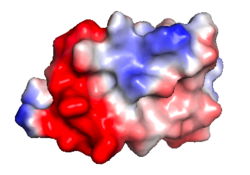
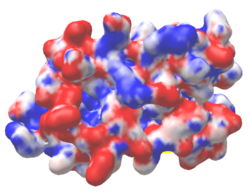
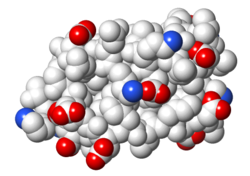
| Protein 1pgb is in the same orientation in all images. Positive + / Negative - | ||
|---|---|---|
| 
| 
|
| Electrostatic potential map rendered by PyMOL. | Electrostatic potential map rendered by iCn3D. | Van der Waals model colored by charge wtih FirstGlance in Jmol. Sidechain nitrogens on Arg/Lys; oxygens on Asp/Glu. |
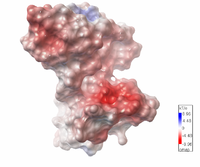
| Electrostatic potential map of 1tsj made with the Embedded Python Molecular Viewer from the Center for Computational Structural Biology of the Scripps Research Institute.
Click on the image to enlarge. |
Methods
PyMOL
- Enter command "fetch 1pgb".
- Menu: All, Action, remove waters.
- Menu: 1pgb, Action, generate, vacuum electrostatics, protein contact potential (local).
See Also
- Electrostatic interactions in Proteopedia.
- Jmol/Electrostatic potential methods.
- Isopotential Map in Wikipedia
- Delphi Web Server
