We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
DNA
From Proteopedia
(Difference between revisions)
| Line 16: | Line 16: | ||
== Features of a DNA Molecule == | == Features of a DNA Molecule == | ||
=== Double Helix === | === Double Helix === | ||
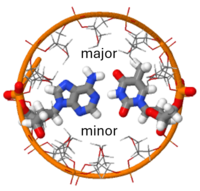
| - | <scene name='User:Adithya_Sagar/Sandbox_DNA/B-dna/4'>DNA</scene> consists of two polynucleotide chains, <scene name='DNA/B-dna/16'>twisted around each other to form a double helix</scene>. The <scene name='10/100853/Nucleotide/2'>nucleotide</scene> in DNA is composed of a <scene name='10/100853/Phosphate/3'>phosphate</scene> bonded to the 5' of <scene name='10/100853/Deoxyribose/2'>D-2'-deoxyribose</scene> which is connected by a beta-glycosidic bond to a purine or a pyrimidine <scene name='10/100853/Base/2'>base</scene>. The <scene name='10/100853/Ribose_pucker/3'>ring pucker</scene> of ribose is a main determinant of which of the [[Forms of DNA]] is present. In this scene, which shows B DNA, the 2' carbon is out of the plane of the other members of the five membered ring. In <scene name='10/100853/3_endo_a_dna/2'>A form DNA</scene>, the 3' carbon is out of the plane of the ribose ring. | + | <scene name='User:Adithya_Sagar/Sandbox_DNA/B-dna/4'>DNA</scene> consists of two polynucleotide chains, <scene name='DNA/B-dna/16'>twisted around each other to form a double helix</scene>. The <scene name='10/100853/Nucleotide/2'>nucleotide</scene> in DNA is composed of a <scene name='10/100853/Phosphate/3'>phosphate</scene> bonded to the 5' of <scene name='10/100853/Deoxyribose/2'>D-2'-deoxyribose</scene> which is connected by a beta-glycosidic bond to a purine or a pyrimidine <scene name='10/100853/Base/2'>base</scene>. The <scene name='10/100853/Ribose_pucker/3'>ring pucker</scene> of ribose is a main determinant of which of the [[Forms of DNA]] is present (see [[Sugar ring pucker]]). In this scene, which shows B DNA, the 2' carbon is out of the plane of the other members of the five membered ring. In <scene name='10/100853/3_endo_a_dna/2'>A form DNA</scene>, the 3' carbon is out of the plane of the ribose ring. |
The four types of bases are the two double-ringed purine base <scene name='10/100853/B-dna/38'>Adenine (A)</scene> and <scene name='10/100853/B-dna/39'>Guanine (G)</scene> and the two single-ringed pyrimidine bases <scene name='10/100853/B-dna/40'>Thymine (T)</scene> and <scene name='10/100853/B-dna/41'>Cytosine (C)</scene>. Hydrogen atoms on some nitrogen and oxygen atom can undergo tautomeric shifts. The nitrogen atoms that are involved in forming tautomer appear as amino or imino groups and the oxygen atoms are either in keto or enol forms. Using an isolate thymine to illustrate the <scene name='DNA/Thymine_enol/1'>imino/enol tautomer</scene> and the <scene name='DNA/Thymine_keto/3'>amino/keto tautomer</scene>. There is a preference for the amino and keto forms which is very crucial for the biological functioning of DNA as it provides a <scene name='10/100853/Amino-glycosidic/2'>ring nitrogen capable of forming a glycosidic bond</scene> with the deoxyribose and it leads to the specificity of hydrogen bonding in base pairing and thus complementarity of the chains.<ref name='Watson'> Watson, James D, Nancy H. Hopkins, Jeffrey W. Roberts, Joan Argetsinger Steitz, Alan M.Weiner ''Molecular Biology of Gene'' (4th ed.). The Benjamin/Cummings Publishing Company Inc.pp. 239-249. ISBN 0-8053-9612-8</ref> The imino nitrogen can only serve as a donating atom in hydrogen bonding, but the amino nitrogen can also serve as a receiving atom. Each nucleotide in a DNA chain is linked to another via <scene name='10/100853/Diester/3'>3',5' phosphodiester bond</scene>. There are four nucleotides in DNA. The sugar-phosphate backbone of the DNA is very regular owing to the phosphodiester linkage whereas the ordering of bases is highly irregular.<ref name='Watson'> Watson, James D, Nancy H. Hopkins, Jeffrey W. Roberts, Joan Argetsinger Steitz, Alan M.Weiner ''Molecular Biology of Gene'' (4th ed.). The Benjamin/Cummings Publishing Company Inc.pp. 239-249. ISBN 0-8053-9612-8</ref> | The four types of bases are the two double-ringed purine base <scene name='10/100853/B-dna/38'>Adenine (A)</scene> and <scene name='10/100853/B-dna/39'>Guanine (G)</scene> and the two single-ringed pyrimidine bases <scene name='10/100853/B-dna/40'>Thymine (T)</scene> and <scene name='10/100853/B-dna/41'>Cytosine (C)</scene>. Hydrogen atoms on some nitrogen and oxygen atom can undergo tautomeric shifts. The nitrogen atoms that are involved in forming tautomer appear as amino or imino groups and the oxygen atoms are either in keto or enol forms. Using an isolate thymine to illustrate the <scene name='DNA/Thymine_enol/1'>imino/enol tautomer</scene> and the <scene name='DNA/Thymine_keto/3'>amino/keto tautomer</scene>. There is a preference for the amino and keto forms which is very crucial for the biological functioning of DNA as it provides a <scene name='10/100853/Amino-glycosidic/2'>ring nitrogen capable of forming a glycosidic bond</scene> with the deoxyribose and it leads to the specificity of hydrogen bonding in base pairing and thus complementarity of the chains.<ref name='Watson'> Watson, James D, Nancy H. Hopkins, Jeffrey W. Roberts, Joan Argetsinger Steitz, Alan M.Weiner ''Molecular Biology of Gene'' (4th ed.). The Benjamin/Cummings Publishing Company Inc.pp. 239-249. ISBN 0-8053-9612-8</ref> The imino nitrogen can only serve as a donating atom in hydrogen bonding, but the amino nitrogen can also serve as a receiving atom. Each nucleotide in a DNA chain is linked to another via <scene name='10/100853/Diester/3'>3',5' phosphodiester bond</scene>. There are four nucleotides in DNA. The sugar-phosphate backbone of the DNA is very regular owing to the phosphodiester linkage whereas the ordering of bases is highly irregular.<ref name='Watson'> Watson, James D, Nancy H. Hopkins, Jeffrey W. Roberts, Joan Argetsinger Steitz, Alan M.Weiner ''Molecular Biology of Gene'' (4th ed.). The Benjamin/Cummings Publishing Company Inc.pp. 239-249. ISBN 0-8053-9612-8</ref> | ||
<scene name='DNA/B-dna/17'>Restore View</scene> | <scene name='DNA/B-dna/17'>Restore View</scene> | ||
Current revision
This page, as it appeared on August 20, 2011, was featured in this article in the journal Biochemistry and Molecular Biology Education.
| |||||||||||
See Also
Proteopedia Articles
- Forms of DNA
- Kinks vs. Bends in DNA are discussed in Lac repressor.
- DNA bulges occur when a nucleotide is inserted in one strand but not the other, causing an interruption in base pairing.
- 1ply
- DNA Replication, Repair, and Recombination - Articles in Proteopedia concerning DNA Replication, Repair, and/or Recombination
- DNA Replication,Transcription and Translation
- Z-DNA
- Transfer ribonucleic acid (tRNA)
- For additional information, see: Nucleic Acids
External Resources
- DNA.MolviZ.Org, an interactive DNA Structure tutorial that is available in nine languages.
- DNA / RNA Section of the Atlas of Macromolecules.
Interpretation of X-Ray Diffraction by DNA
- Anatomy of Photo 51, Rosalind Franklin's diffraction pattern used by Watson & Crick in developing their model of the DNA double helix (at PBS.Org, US Public Broadcasting System).
- Explanation of Franklin's X-Ray Diffraction Pattern at Cold Spring Harbor Laboratory, USA.
- More technical: How Rosalind Franklin Discovered the Helical Structure of DNA: Experiments in Diffraction.
References
- ↑ 1.0 1.1 http://www.genome.gov/25520880
- ↑ Dahm R. Discovering DNA: Friedrich Miescher and the early years of nucleic acid research. Hum Genet. 2008 Jan;122(6):565-81. Epub 2007 Sep 28. PMID:17901982 doi:10.1007/s00439-007-0433-0
- ↑ 3.0 3.1 A Structure for Deoxyribose Nucleic Acid Watson J.D. and Crick F.H.C. Nature 171, 737-738 (1953)
- ↑ 4.0 4.1 4.2 Watson, James D, Nancy H. Hopkins, Jeffrey W. Roberts, Joan Argetsinger Steitz, Alan M.Weiner Molecular Biology of Gene (4th ed.). The Benjamin/Cummings Publishing Company Inc.pp. 239-249. ISBN 0-8053-9612-8
- ↑ SantaLucia J Jr. A unified view of polymer, dumbbell, and oligonucleotide DNA nearest-neighbor thermodynamics. Proc Natl Acad Sci U S A. 1998 Feb 17;95(4):1460-5. PMID:9465037
- ↑ Saenger, Wolfram (1984). Principles of Nucleic Acid Structure (1st ed). Springer-Verlag. pp. 398. ISBN 0-12-645750-6.
- ↑ Rawn,David J. "Biochemistry"(1st ed.) Harper&Row,Publishers, Inc.pp. 1024-1050. ISBN-0-06045335-4
- ↑ Maddox, Brenda: Rosalind Franklin: Dark Lady of DNA, HarperCollins, 2002
- ↑ Berman HM, Gelbin A, Westbrook J. Nucleic acid crystallography: a view from the nucleic acid database. Prog Biophys Mol Biol. 1996;66(3):255-88. PMID:9284453
- ↑ Chandrasekaran R, Arnott S. The structure of B-DNA in oriented fibers. J Biomol Struct Dyn. 1996 Jun;13(6):1015-27. PMID:8832384
Proteopedia Page Contributors and Editors (what is this?)
Adithya Sagar, Eran Hodis, Ala Jelani, Eric Martz, Wayne Decatur, Karsten Theis, Alexander Berchansky, Karl Oberholser, Joel L. Sussman, Ann Taylor, David Canner, Angel Herraez, Joseph M. Steinberger, Frédéric Dardel