We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1846
From Proteopedia
(Difference between revisions)
| Line 9: | Line 9: | ||
== Function == | == Function == | ||
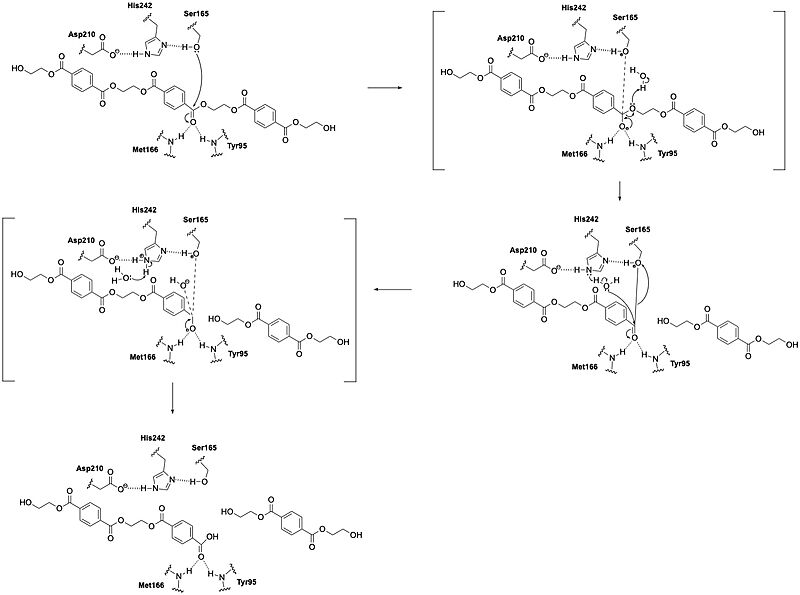
| - | LCC catalyzes the hydrolysis of the ester bonds in | + | LCC catalyzes the hydrolysis of the [https://en.wikipedia.org/wiki/Ester ester] bonds in [https://en.wikipedia.org/wiki/Polymer polymers] of PET and breaks them down into their constituent monomers: [https://en.wikipedia.org/wiki/Terephthalic_acid terephthalic acid] and [https://en.wikipedia.org/wiki/Ethylene_glycol ethylene glycol]. The enzyme operates through a [https://en.wikipedia.org/wiki/Catalytic_triad catalytic triad] that consists of S165, D210, and H242. Using these residues, LCC can perform [https://en.wikipedia.org/wiki/Nucleophile nucleophilic attacks] on the [https://en.wikipedia.org/wiki/Carbonyl_group carbonyl] carbon atoms of the ester bonds in PET. During catalysis, the substrate binds in an elongated, predominantly [https://en.wikipedia.org/wiki/Hydrophobe hydrophobic] groove present in the enzyme's structure. |
| - | LCC functions | + | |
| - | LCC's function is limited by PET [https://en.wikipedia.org/wiki/Crystallization_of_polymers crystallinity], as the enzyme can more effectively hydrolyze amorphous regions of the polymer. As PET crystallinity increases during the depolymerization reaction (due to exposure to elevated temperatures), the enzyme's efficiency decreases. This limits complete depolymerization unless optimal conditions and enzyme variants are used. | + | LCC functions best at elevated temperatures (around 65–72°C), which approaches the [https://en.wikipedia.org/wiki/Glass_transition glass transition] temperature of PET. This temperature range maximizes PET chain mobility and makes the polymer more accessible to enzymatic action. The enzyme is remarkably [https://en.wikipedia.org/wiki/Thermostability thermostable] compared to other PET hydrolases, with a [https://en.wikipedia.org/wiki/Melting_point melting temperature] of 84.7°C. This property allows it to remain functional under these high-temperature conditions. Also unlike other PET hydrolases such as Is-PETase, BTA1, BTA2, and FsC, LCC has substantially higher [https://en.wikipedia.org/wiki/Enzyme_efficiency catalytic efficiency]. Specifically, LCC has an initial PET-specific [https://en.wikipedia.org/wiki/Depolymerization depolymerization] rate of 93.2 mg TAeq·h⁻¹·mg⁻¹ enzyme at 65°C with [https://en.wikipedia.org/wiki/Amorphous_solid amorphous] PET. This means that it is at least 33 times more efficient than other tested enzymes.<ref name="Tournier">PMID:32269349</ref> LCC's function is limited by PET [https://en.wikipedia.org/wiki/Crystallization_of_polymers crystallinity], as the enzyme can more effectively hydrolyze amorphous regions of the polymer. As PET crystallinity increases during the depolymerization reaction (due to exposure to elevated temperatures), the enzyme's efficiency decreases. This limits complete depolymerization unless optimal conditions and enzyme variants are used. |
== Relevance == | == Relevance == | ||
| - | With global plastic production reaching approximately 299 million tons annually, the need for effective waste management solutions is urgent. Enzymatic degradation is an alternative to conventional recycling methods that are often inefficient and taxing on the environment. One of the primary challenges in plastic waste management is the volume of mismanaged plastic entering marine environments. In 2010 alone, an estimated 31.9 million metric tons of plastic waste were classified as mismanaged, with a substantial portion ending up in the | + | With global [https://en.wikipedia.org/wiki/Plastic_production plastic production] reaching approximately 299 million tons annually, the need for effective [https://en.wikipedia.org/wiki/Waste_management waste management] solutions is urgent. [https://en.wikipedia.org/wiki/Enzymatic_biodegradation Enzymatic degradation] is an alternative to conventional [https://en.wikipedia.org/wiki/Plastic_recycling recycling] methods that are often inefficient and taxing on the environment. One of the primary challenges in plastic waste management is the volume of [https://en.wikipedia.org/wiki/Plastic_pollution mismanaged plastic] entering [https://en.wikipedia.org/wiki/Marine_environment marine environments]. In 2010 alone, an estimated 31.9 million metric tons of plastic waste were classified as mismanaged, with a substantial portion ending up in the [https://en.wikipedia.org/wiki/Ocean ocean]s. This causes harm to [https://en.wikipedia.org/wiki/Marine_ecosystem marine ecosystems], physical injury to [https://en.wikipedia.org/wiki/Marine_life wildlife], and disruption of [https://en.wikipedia.org/wiki/Food_chain food chains].<ref name="Landrigan">PMID:33354517</ref> |
| - | Integrating LCC into existing waste management systems could substantially reduce the PET waste that enters the environment. Research suggests that a 77% reduction in mismanaged plastic waste could lower the annual input of plastic into the ocean to between 2.4 and 6.4 million metric tons by 2025. | + | |
| - | LCC hydrolyzes PET into its constituent monomers, which also supports the principles of a circular economy, where materials are reused rather than discarded. Enzymatic degradation allows for the production of biologically recycled PET with properties that are comparable to virgin materials.<ref name="Jambeck">PMID:25678662</ref> | + | Integrating LCC into existing waste management systems could substantially reduce the PET waste that enters the environment. Research suggests that a 77% reduction in mismanaged plastic waste could lower the annual input of plastic into the ocean to between 2.4 and 6.4 million metric tons by 2025. LCC hydrolyzes PET into its constituent monomers, which also supports the principles of a [https://en.wikipedia.org/wiki/Circular_economy circular economy], where materials are reused rather than discarded. Enzymatic degradation allows for the production of [https://en.wikipedia.org/wiki/Bioplastic biologically recycled] PET with properties that are comparable to [https://en.wikipedia.org/wiki/Plastic#Virgin_plastic virgin materials].<ref name="Jambeck">PMID:25678662</ref> |
== Structural Overview == | == Structural Overview == | ||
Revision as of 19:52, 14 April 2025
| This Sandbox is Reserved from March 18 through September 1, 2025 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson and Mark Macbeth at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1828 through Sandbox Reserved 1846. |
To get started:
More help: Help:Editing |
Leaf Branch Compost Cutinase
| |||||||||||
References
- ↑ 1.0 1.1 Tournier V, Topham CM, Gilles A, David B, Folgoas C, Moya-Leclair E, Kamionka E, Desrousseaux ML, Texier H, Gavalda S, Cot M, Guemard E, Dalibey M, Nomme J, Cioci G, Barbe S, Chateau M, Andre I, Duquesne S, Marty A. An engineered PET depolymerase to break down and recycle plastic bottles. Nature. 2020 Apr;580(7802):216-219. doi: 10.1038/s41586-020-2149-4. Epub 2020 Apr, 8. PMID:32269349 doi:http://dx.doi.org/10.1038/s41586-020-2149-4
- ↑ Kolattukudy PE. Biopolyester membranes of plants: cutin and suberin. Science. 1980 May 30;208(4447):990-1000. PMID:17779010 doi:10.1126/science.208.4447.990
- ↑ Burgin T, Pollard BC, Knott BC, Mayes HB, Crowley MF, McGeehan JE, Beckham GT, Woodcock HL. The reaction mechanism of the Ideonella sakaiensis PETase enzyme. Commun Chem. 2024 Mar 27;7(1):65. PMID:38538850 doi:10.1038/s42004-024-01154-x
- ↑ Landrigan PJ, Stegeman JJ, Fleming LE, Allemand D, Anderson DM, Backer LC, Brucker-Davis F, Chevalier N, Corra L, Czerucka D, Bottein MD, Demeneix B, Depledge M, Deheyn DD, Dorman CJ, Fénichel P, Fisher S, Gaill F, Galgani F, Gaze WH, Giuliano L, Grandjean P, Hahn ME, Hamdoun A, Hess P, Judson B, Laborde A, McGlade J, Mu J, Mustapha A, Neira M, Noble RT, Pedrotti ML, Reddy C, Rocklöv J, Scharler UM, Shanmugam H, Taghian G, van de Water JAJM, Vezzulli L, Weihe P, Zeka A, Raps H, Rampal P. Human Health and Ocean Pollution. Ann Glob Health. 2020 Dec 3;86(1):151. PMID:33354517 doi:10.5334/aogh.2831
- ↑ Jambeck JR, Geyer R, Wilcox C, Siegler TR, Perryman M, Andrady A, Narayan R, Law KL. Marine pollution. Plastic waste inputs from land into the ocean. Science. 2015 Feb 13;347(6223):768-71. PMID:25678662 doi:10.1126/science.1260352
Student Contributors
Ashley Callaghan Rebecca Hoff Simone McCowan