We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1845
From Proteopedia
(Difference between revisions)
| Line 47: | Line 47: | ||
<scene name='10/1075247/Y127/4'>Y127</scene> | <scene name='10/1075247/Y127/4'>Y127</scene> | ||
| - | <scene name='10/1075247/Y127g/ | + | <scene name='10/1075247/Y127g/2'>Y127G</scene> |
The mutation of tyrosine to glycine at position 127 (Y127G) also increases the protein's thermostability. The mutant melting point is increased to 87.0°C. Tyrosine has a bulky, rigid aromatic side chain that can cause structural strain. Glycine is the smallest amino acid and lacks a side chain, so it provides greater flexibility to the protein. This mutation reduces steric hindrance and relieves strain in the protein structure, therefore allowing it to be more adaptable and stable at higher temperatures. By increasing flexibility, the Y127G mutation helps the protein maintain its folded structure under heat stress. | The mutation of tyrosine to glycine at position 127 (Y127G) also increases the protein's thermostability. The mutant melting point is increased to 87.0°C. Tyrosine has a bulky, rigid aromatic side chain that can cause structural strain. Glycine is the smallest amino acid and lacks a side chain, so it provides greater flexibility to the protein. This mutation reduces steric hindrance and relieves strain in the protein structure, therefore allowing it to be more adaptable and stable at higher temperatures. By increasing flexibility, the Y127G mutation helps the protein maintain its folded structure under heat stress. | ||
Revision as of 22:20, 14 April 2025
| This Sandbox is Reserved from March 18 through September 1, 2025 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson and Mark Macbeth at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1828 through Sandbox Reserved 1846. |
To get started:
More help: Help:Editing |
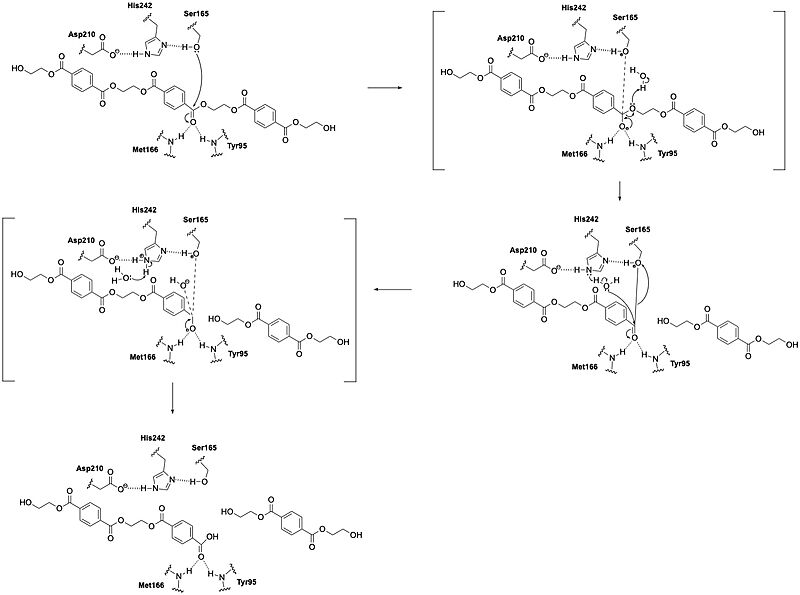
Leaf Branch Compost Cutinase
| |||||||||||
References
- ↑ 1.0 1.1 Tournier V, Topham CM, Gilles A, David B, Folgoas C, Moya-Leclair E, Kamionka E, Desrousseaux ML, Texier H, Gavalda S, Cot M, Guemard E, Dalibey M, Nomme J, Cioci G, Barbe S, Chateau M, Andre I, Duquesne S, Marty A. An engineered PET depolymerase to break down and recycle plastic bottles. Nature. 2020 Apr;580(7802):216-219. doi: 10.1038/s41586-020-2149-4. Epub 2020 Apr, 8. PMID:32269349 doi:http://dx.doi.org/10.1038/s41586-020-2149-4
- ↑ Kolattukudy PE. Biopolyester membranes of plants: cutin and suberin. Science. 1980 May 30;208(4447):990-1000. PMID:17779010 doi:10.1126/science.208.4447.990
- ↑ Burgin T, Pollard BC, Knott BC, Mayes HB, Crowley MF, McGeehan JE, Beckham GT, Woodcock HL. The reaction mechanism of the Ideonella sakaiensis PETase enzyme. Commun Chem. 2024 Mar 27;7(1):65. PMID:38538850 doi:10.1038/s42004-024-01154-x
- ↑ Landrigan PJ, Stegeman JJ, Fleming LE, Allemand D, Anderson DM, Backer LC, Brucker-Davis F, Chevalier N, Corra L, Czerucka D, Bottein MD, Demeneix B, Depledge M, Deheyn DD, Dorman CJ, Fénichel P, Fisher S, Gaill F, Galgani F, Gaze WH, Giuliano L, Grandjean P, Hahn ME, Hamdoun A, Hess P, Judson B, Laborde A, McGlade J, Mu J, Mustapha A, Neira M, Noble RT, Pedrotti ML, Reddy C, Rocklöv J, Scharler UM, Shanmugam H, Taghian G, van de Water JAJM, Vezzulli L, Weihe P, Zeka A, Raps H, Rampal P. Human Health and Ocean Pollution. Ann Glob Health. 2020 Dec 3;86(1):151. PMID:33354517 doi:10.5334/aogh.2831
- ↑ Jambeck JR, Geyer R, Wilcox C, Siegler TR, Perryman M, Andrady A, Narayan R, Law KL. Marine pollution. Plastic waste inputs from land into the ocean. Science. 2015 Feb 13;347(6223):768-71. PMID:25678662 doi:10.1126/science.1260352
Student Contributors
Ashley Callaghan Rebecca Hoff Simone McCowan