We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Emily Hwang/Sandbox1
From Proteopedia
< User:Emily Hwang(Difference between revisions)
| Line 12: | Line 12: | ||
<scene name='10/1075193/Wild_type_pet_hydrolase/8'>Leaf-branch compost cutinase</scene>, LCC, is a part of the [https://en.wikipedia.org/wiki/Serine_hydrolase serine hydrolase] family. It is a monomer that contains a total of 258 amino acid residues, with an amphipathic structure. It has a <scene name='10/1075193/Overall_topology/3'>secondary structure</scene> made up of alpha helices and beta turns, which correlate to the alpha-beta hydrolase family. The active site consists of a <scene name='10/1075191/Active_site_overall_topology/9'>catalytic triad</scene>, which is a common feature among serine hydrolases. Compared to other serine hydrolases and cutinases studied for plastic degradation, the LCC proved to be 33x more efficient. <ref name="Tournier et. al. 2020">PMID:32269349</ref> | <scene name='10/1075193/Wild_type_pet_hydrolase/8'>Leaf-branch compost cutinase</scene>, LCC, is a part of the [https://en.wikipedia.org/wiki/Serine_hydrolase serine hydrolase] family. It is a monomer that contains a total of 258 amino acid residues, with an amphipathic structure. It has a <scene name='10/1075193/Overall_topology/3'>secondary structure</scene> made up of alpha helices and beta turns, which correlate to the alpha-beta hydrolase family. The active site consists of a <scene name='10/1075191/Active_site_overall_topology/9'>catalytic triad</scene>, which is a common feature among serine hydrolases. Compared to other serine hydrolases and cutinases studied for plastic degradation, the LCC proved to be 33x more efficient. <ref name="Tournier et. al. 2020">PMID:32269349</ref> | ||
=Active Site= | =Active Site= | ||
| - | ==Structure== | + | ==Structure== --Emily changed this |
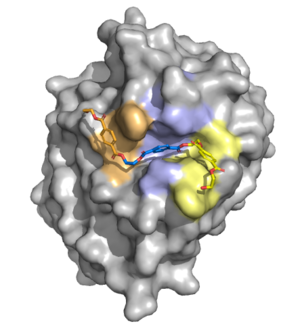
The active site contains a hydrophobic binding pocket which makes [https://en.wikipedia.org/wiki/Pi-stacking aromatic pi-stacking] and [https://en.wikipedia.org/wiki/Van_der_Waals_force Van der Waals interactions] with the aromatic rings in the PET ligand. These interactions stabilize the binding of the PET polymer within the enzyme’s active site and orient it for catalysis. <b>Figure 1</b> illustrates a surface model of the ligand bound to the hydrophobic binding pocket. | The active site contains a hydrophobic binding pocket which makes [https://en.wikipedia.org/wiki/Pi-stacking aromatic pi-stacking] and [https://en.wikipedia.org/wiki/Van_der_Waals_force Van der Waals interactions] with the aromatic rings in the PET ligand. These interactions stabilize the binding of the PET polymer within the enzyme’s active site and orient it for catalysis. <b>Figure 1</b> illustrates a surface model of the ligand bound to the hydrophobic binding pocket. | ||
{| class="wikitable" style="width: 50%; float:right; margin-left: 1em;" | {| class="wikitable" style="width: 50%; float:right; margin-left: 1em;" | ||
| Line 53: | Line 53: | ||
[[Image:Surf1.png|300 px|center|thumb|<b>Figure 1.</b> Surface rendering of the enzyme showing the hydrophobic binding pocket. Residues are color-coded based on the monomer they are interacting with in the PET substrate.]] | [[Image:Surf1.png|300 px|center|thumb|<b>Figure 1.</b> Surface rendering of the enzyme showing the hydrophobic binding pocket. Residues are color-coded based on the monomer they are interacting with in the PET substrate.]] | ||
<br style="clear:both;"> | <br style="clear:both;"> | ||
| + | |||
==Mechanism== | ==Mechanism== | ||
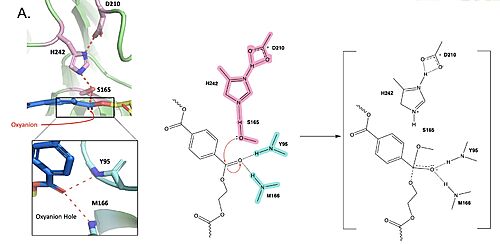
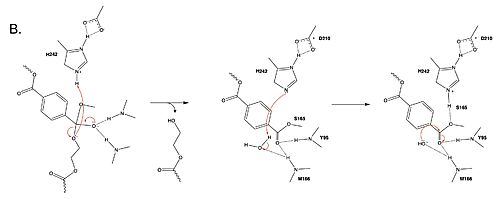
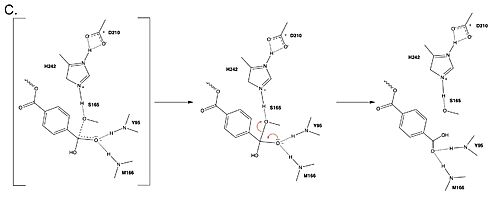
Polyethylene terephthalate (PET) hydrolase (PETase) is a serine hydrolase that catalyzes the cleavage of ester bonds in PET polymers. <center>[[Image:PartImechaism.jpg|500 px|center|thumb|Figure 1. Active site of PETase highlighting catalytic residues and the first step of the reaction mechanism. Residues in pink represent the catalytic triad (S165, H242, and D210). Light blue represents the oxyanion hole residues (Y95 and M166).]]</center>The mechanism of the PET Hydrolase involves a proton relay by the <scene name='10/1075193/Cat_triad_ligand/5'>catalytic triad</scene>, making the catalytic S165 a good nucleophile. S165 attacks the carbonyl carbon in the -1 monomer of the PET Polymer, forming a tetrahedral intermediate. The pi electrons move onto the carbonyl oxygen, creating an oxyanion which is stabilized by the <scene name='10/1075193/Oxyanion_hole/4'>oxyanion hole</scene>, consisting of the backbone amide nitrogens of Y95 and M166. Then, the leaving group oxygen on the -2 monomer is protonated by H242, which is also a part of the catalytic triad. This facilitates the reformation of the carbonyl group upon collapse of the oxyanion and the severing of the <scene name='10/1075193/Scissile_bond/4'>scissile bond</scene>. | Polyethylene terephthalate (PET) hydrolase (PETase) is a serine hydrolase that catalyzes the cleavage of ester bonds in PET polymers. <center>[[Image:PartImechaism.jpg|500 px|center|thumb|Figure 1. Active site of PETase highlighting catalytic residues and the first step of the reaction mechanism. Residues in pink represent the catalytic triad (S165, H242, and D210). Light blue represents the oxyanion hole residues (Y95 and M166).]]</center>The mechanism of the PET Hydrolase involves a proton relay by the <scene name='10/1075193/Cat_triad_ligand/5'>catalytic triad</scene>, making the catalytic S165 a good nucleophile. S165 attacks the carbonyl carbon in the -1 monomer of the PET Polymer, forming a tetrahedral intermediate. The pi electrons move onto the carbonyl oxygen, creating an oxyanion which is stabilized by the <scene name='10/1075193/Oxyanion_hole/4'>oxyanion hole</scene>, consisting of the backbone amide nitrogens of Y95 and M166. Then, the leaving group oxygen on the -2 monomer is protonated by H242, which is also a part of the catalytic triad. This facilitates the reformation of the carbonyl group upon collapse of the oxyanion and the severing of the <scene name='10/1075193/Scissile_bond/4'>scissile bond</scene>. | ||
Current revision
| |||||||||||
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 Tournier V, Topham CM, Gilles A, David B, Folgoas C, Moya-Leclair E, Kamionka E, Desrousseaux ML, Texier H, Gavalda S, Cot M, Guemard E, Dalibey M, Nomme J, Cioci G, Barbe S, Chateau M, Andre I, Duquesne S, Marty A. An engineered PET depolymerase to break down and recycle plastic bottles. Nature. 2020 Apr;580(7802):216-219. doi: 10.1038/s41586-020-2149-4. Epub 2020 Apr, 8. PMID:32269349 doi:http://dx.doi.org/10.1038/s41586-020-2149-4
- ↑ 2.0 2.1 Boneta S, Arafet K, Moliner V. QM/MM Study of the Enzymatic Biodegradation Mechanism of Polyethylene Terephthalate. J Chem Inf Model. 2021 Jun 28;61(6):3041-3051. PMID:34085821 doi:10.1021/acs.jcim.1c00394
- ↑ 3.0 3.1 3.2 3.3 3.4 Shirke AN, White C, Englaender JA, Zwarycz A, Butterfoss GL, Linhardt RJ, Gross RA. Stabilizing Leaf and Branch Compost Cutinase (LCC) with Glycosylation: Mechanism and Effect on PET Hydrolysis. Biochemistry. 2018 Feb 20;57(7):1190-1200. PMID:29328676 doi:10.1021/acs.biochem.7b01189
- ↑ 4.0 4.1 4.2 . PMID:330311984
- ↑ Lord CC, Thomas G, Brown JM. Mammalian alpha beta hydrolase domain (ABHD) proteins: Lipid metabolizing enzymes at the interface of cell signaling and energy metabolism. Biochim Biophys Acta. 2013 Apr;1831(4):792-802. PMID:23328280 doi:10.1016/j.bbalip.2013.01.002
- ↑ Imperiali B, O'Connor SE. Effect of N-linked glycosylation on glycopeptide and glycoprotein structure. Curr Opin Chem Biol. 1999 Dec;3(6):643-9. PMID:10600722
- ↑ The sodium sulfate dilemma: The unforeseen challenge of lithium battery recycling.[1] (2023).
- ↑ Recycle: Promote “Bottle to Bottle” Horizontal Recycling.[2]
Student Contributors
- Georgia Apple
- Emily Hwang
- Anjali Rabindran