We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1852
From Proteopedia
(Difference between revisions)
| Line 30: | Line 30: | ||
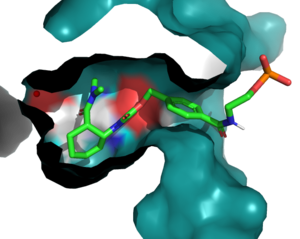
<scene name='10/1075254/N285/1'>Residue 285</scene>, as follows, is also buried within the binding pocket. The group introduced this mutation to increase steric hindrance with the catalytic tyrosine, reducing the number of rotamers the residue has to increase the reactivity of the enzyme by lowering the distance between Y134 and the ligand. | <scene name='10/1075254/N285/1'>Residue 285</scene>, as follows, is also buried within the binding pocket. The group introduced this mutation to increase steric hindrance with the catalytic tyrosine, reducing the number of rotamers the residue has to increase the reactivity of the enzyme by lowering the distance between Y134 and the ligand. | ||
===CE6=== | ===CE6=== | ||
| - | The DA_20_10 model of the Diels Alderase was further enhanced by players of the online game "Foldit." Building on preliminary early data, players were asked to optimize various helical structures that would surround and support the ligand. After over 100,000 designs were tested, the top-scoring CE6 model was finalized, containing as alpha helix cap that favorably constrains ligand orientation. This "cap" consists of two helices--helix one spans from residues 36-44, and helix two spans from residues 48-56. | + | The DA_20_10 model of the Diels Alderase was further enhanced by players of the online game "Foldit." Building on preliminary early data, players were asked to optimize various helical structures that would surround and support the ligand. After over 100,000 designs were tested, the top-scoring CE6 model was finalized, containing as <scene name='10/1075252/Alpha_helix_highlighted/4'>alpha helix cap</scene> that favorably constrains ligand orientation. This "cap" consists of two helices--helix one spans from residues 36-44, and helix two spans from residues 48-56. |
===CE20=== | ===CE20=== | ||
In this generation, it was found that the most catalytically efficient models had mutated T34, P48, and R56 to <scene name='10/1075254/Ce_20_mutations/4'>I43,L48, and S56</scene>. These mutations further tightened the binding pocket and create a more hydrophobic environment. | In this generation, it was found that the most catalytically efficient models had mutated T34, P48, and R56 to <scene name='10/1075254/Ce_20_mutations/4'>I43,L48, and S56</scene>. These mutations further tightened the binding pocket and create a more hydrophobic environment. | ||
| + | ===Kinetics=== | ||
| + | [[Image:Diels_Alderase_kinetic_table_Large.jpeg|300px|left]] | ||
| + | Catalytic efficiency of 4 key generations of Diels-Alderase enzyme. Kinetic data was measured at 25 degrees Celsius, in PBS, at pH 7.4 (cite 3) | ||
==Application== | ==Application== | ||
Revision as of 18:10, 15 April 2025
| This Sandbox is Reserved from March 18 through September 1, 2025 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson and Mark Macbeth at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1828 through Sandbox Reserved 1846. |
To get started:
More help: Help:Editing |
Diels-Alderase
| |||||||||||
References
- ↑ Ransey E, Paredes E, Dey SK, Das SR, Heroux A, Macbeth MR. Crystal structure of the Entamoeba histolytica RNA lariat debranching enzyme EhDbr1 reveals a catalytic Zn(2+) /Mn(2+) heterobinucleation. FEBS Lett. 2017 Jul;591(13):2003-2010. doi: 10.1002/1873-3468.12677. Epub 2017, Jun 14. PMID:28504306 doi:http://dx.doi.org/10.1002/1873-3468.12677
Student Contributors
Taylor Donahue Kate Thuma Micah Zile