User:Hayden Vissing/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 20: | Line 20: | ||
===Overview of Active Site=== | ===Overview of Active Site=== | ||
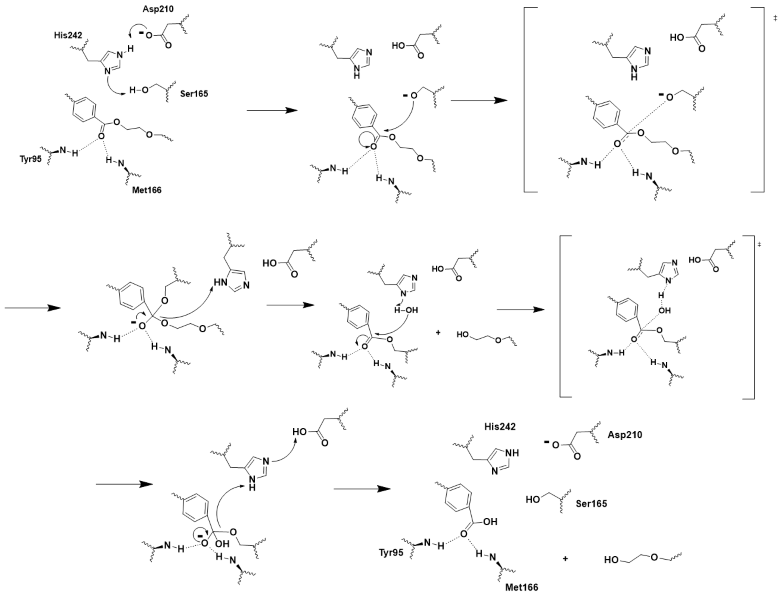
| - | + | LCC is a serine hydrolase. The mechanism uses a <scene name='10/1076051/Wt_cat_triad/3'>catalytic triad</scene>: S165 (which was made into an alanine for crystallization purposes), D210, and H242. S165 is the nucleophile attacking the electrophilic carbonyl carbon with the <scene name='10/1075218/Oxyanion_hole/4'>oxyanion hole</scene> to stabilize the translation state. These key residues of the catalytic triad and oxyanion hole help to make up the <scene name='10/1076051/Wt_active_site/1'>active site</scene>. | |
| - | LCC is a serine hydrolase. The mechanism uses a <scene name='10/1076051/Wt_cat_triad/3'>catalytic triad</scene>: S165 (which was made into an alanine for crystallization purposes), D210, and H242. S165 is the nucleophile attacking the electrophilic carbonyl carbon with the <scene name='10/1075218/Oxyanion_hole/4'>oxyanion hole</scene> to stabilize the translation state. | + | |
[[Image:Push_mechanism.png|800px|center|thumb|Figure 2 - Push-mechanism for the cutinase reaction]] | [[Image:Push_mechanism.png|800px|center|thumb|Figure 2 - Push-mechanism for the cutinase reaction]] | ||
| Line 28: | Line 27: | ||
==Mutations== | ==Mutations== | ||
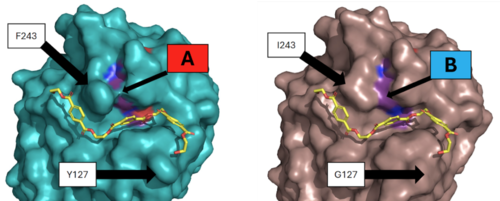
| - | After selection, LCC was mutated in order to improve its efficiency and thermal stability. Tournier et al. (2020) identified <scene name='10/1075218/Wildtype_residues/5'>11 residues</scene> from the wildtype | + | After selection, LCC was mutated in order to improve its efficiency and thermal stability. Tournier et al. (2020) identified <scene name='10/1075218/Wildtype_residues/5'>11 residues</scene> from the wildtype as being apart of the <scene name='10/1076051/Contact_shell/1'>first contact shell</scene>. They then made hundreds of different mutation commutations, marking which altered efficiency and stability the most. Two of these 11 were chosen for the final mutant enzyme, <scene name='10/1075218/Identifiedresidues/12'>F243I and Y127G</scene>. These mutations were paired with two separately identified thermostability specific mutations (D238C and S283C) to make the final <scene name='10/1076051/Mutant_overview_shade/2'>ICCG</scene> enzyme. |
===Efficiency improvement=== | ===Efficiency improvement=== | ||
Revision as of 19:34, 15 April 2025
The Future of Recycling: the ICCG Mutant Pet Hydroalase
| |||||||||||
References
Student Contributors
- David Bogle
- Justin Chavez
- Hayden Vissing