User:Hayden Vissing/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 35: | Line 35: | ||
===Thermostability improvement=== | ===Thermostability improvement=== | ||
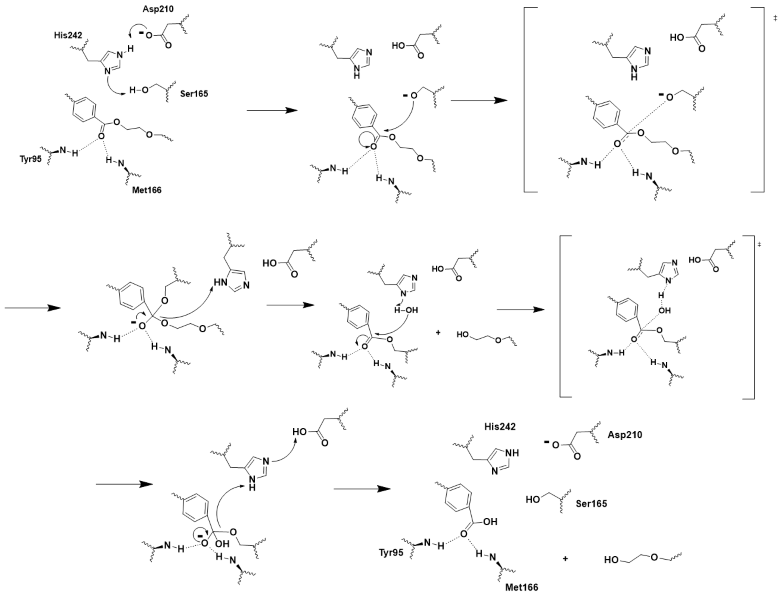
To allow the enzyme to be effective in the recycling of PET, it needed to be stable in the high temperature environments used during the recycling process. Because of this, improvements needed to be made to the thermostability of the enzyme. The researchers went about this by searching for divalent metal binding sites, which had been seen to improve the thermal stability of other PET hydrolase enzymes. They found this site formed by the residues of E208, D238, and S283. These residues formed a similar structure that had been seen in other PET hydrolase enzymes, and when calcium ions were added to the enzyme the thermal stability improved. In order to avoid issues with purification, the team opted to avoid the addition of calcium salts and to instead create a disulfide bridge at the location of the divalent metal binding site. They chose to replace the <scene name='10/1076054/Thermostability_wild_type/3'>aspartic acid at 238 and the serine at 283</scene> with cysteine residues to form a <scene name='10/1076054/Iccg_disulfide_bridge/3'>disulfide bridge</scene>. This mutation improved the melting temperature of the enzyme by 9.8℃. | To allow the enzyme to be effective in the recycling of PET, it needed to be stable in the high temperature environments used during the recycling process. Because of this, improvements needed to be made to the thermostability of the enzyme. The researchers went about this by searching for divalent metal binding sites, which had been seen to improve the thermal stability of other PET hydrolase enzymes. They found this site formed by the residues of E208, D238, and S283. These residues formed a similar structure that had been seen in other PET hydrolase enzymes, and when calcium ions were added to the enzyme the thermal stability improved. In order to avoid issues with purification, the team opted to avoid the addition of calcium salts and to instead create a disulfide bridge at the location of the divalent metal binding site. They chose to replace the <scene name='10/1076054/Thermostability_wild_type/3'>aspartic acid at 238 and the serine at 283</scene> with cysteine residues to form a <scene name='10/1076054/Iccg_disulfide_bridge/3'>disulfide bridge</scene>. This mutation improved the melting temperature of the enzyme by 9.8℃. | ||
| + | |||
| + | ==Conlusions== | ||
| + | |||
| + | ===Resulting data=== | ||
| + | |||
| + | ===Future directions=== | ||
</StructureSection> | </StructureSection> | ||
Revision as of 19:54, 15 April 2025
The Future of Recycling: the ICCG Mutant Pet Hydroalase
| |||||||||||
References
Student Contributors
- David Bogle
- Justin Chavez
- Hayden Vissing